For the treatment of adult patients with moderately to severely active CD
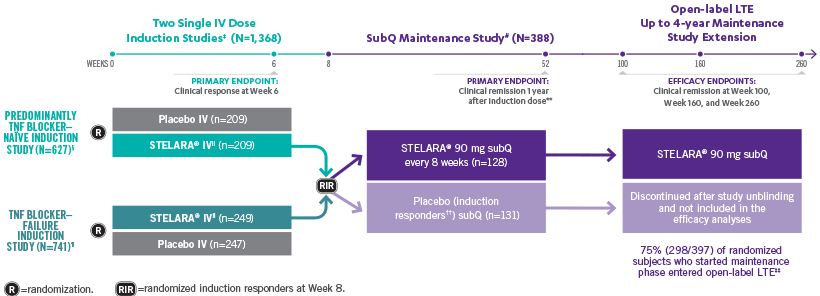
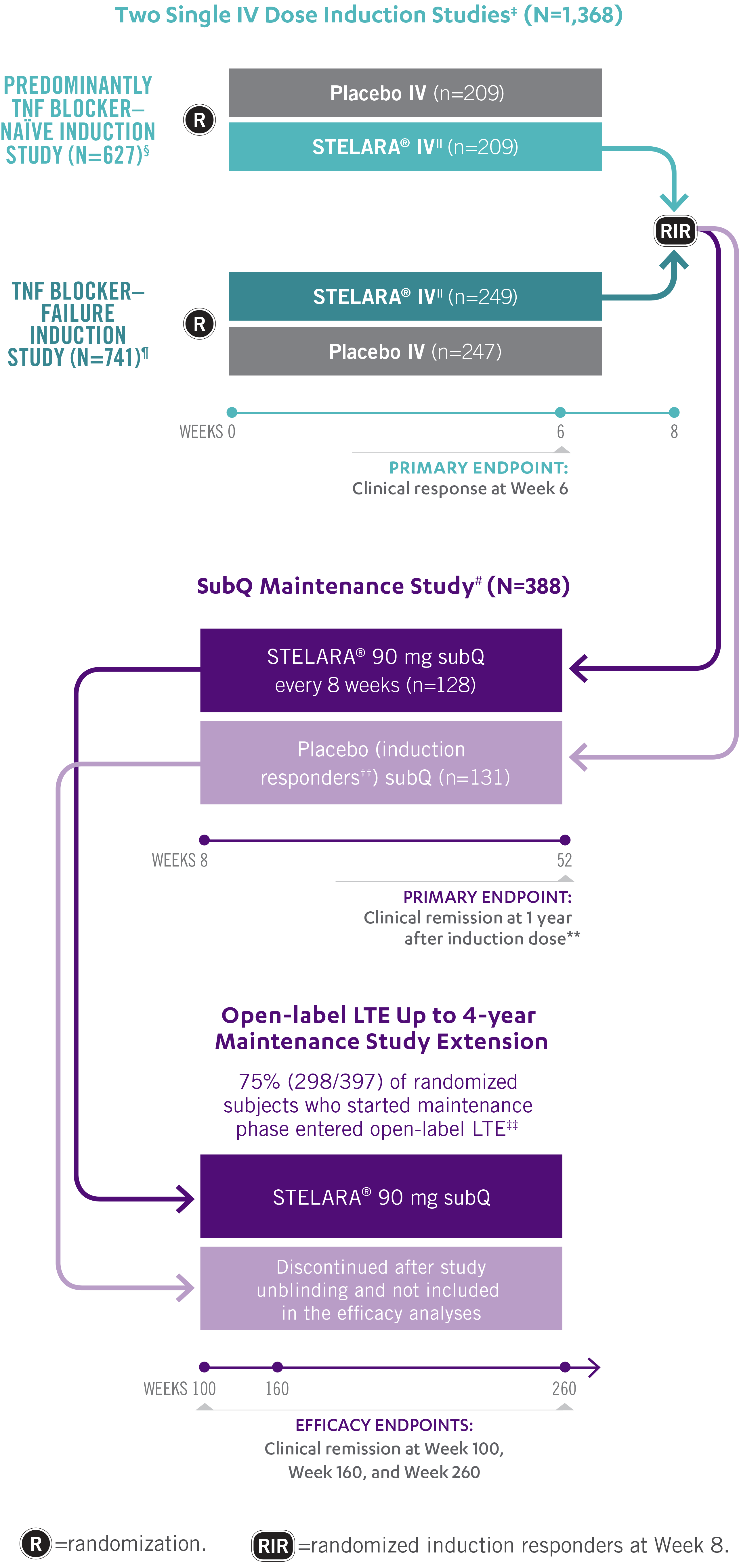
STELARA®: FAST
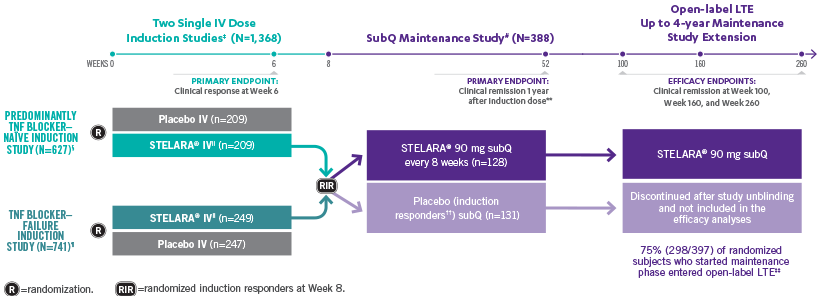
INDUCTION STUDY
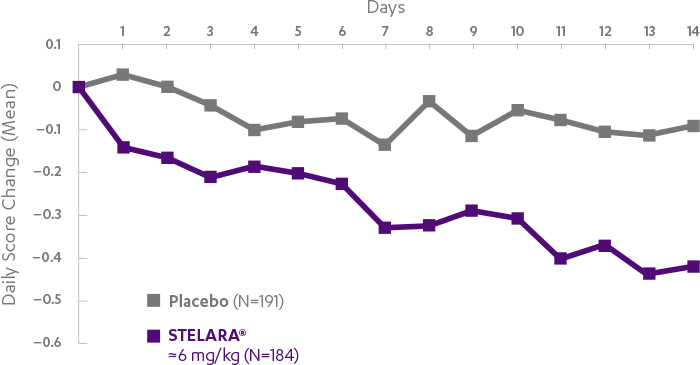
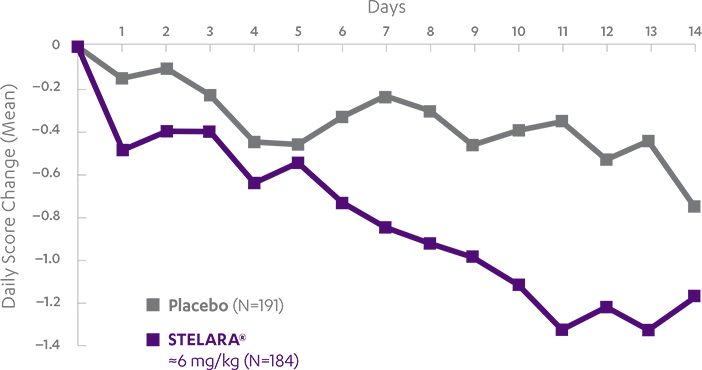
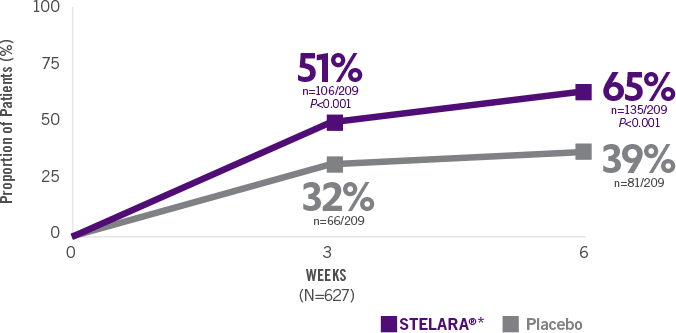
STELARA®*DELIVERED RAPID RESPONSE AS EARLY AS WEEK 6 (CLINICAL RESPONSE) AND WEEK 3 (70-POINT RESPONSE)†
Clinical response was defined as reduction in CDAI score of ≥100 points or CDAI score of <1501
Significantly greater proportion of predominantly TNF blocker–naïve patients achieved 70-point response† at Week 3
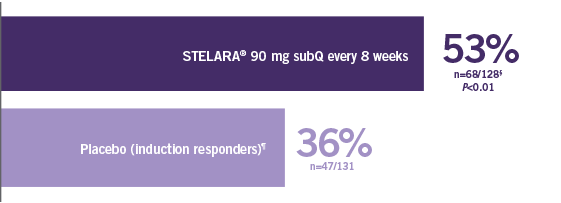
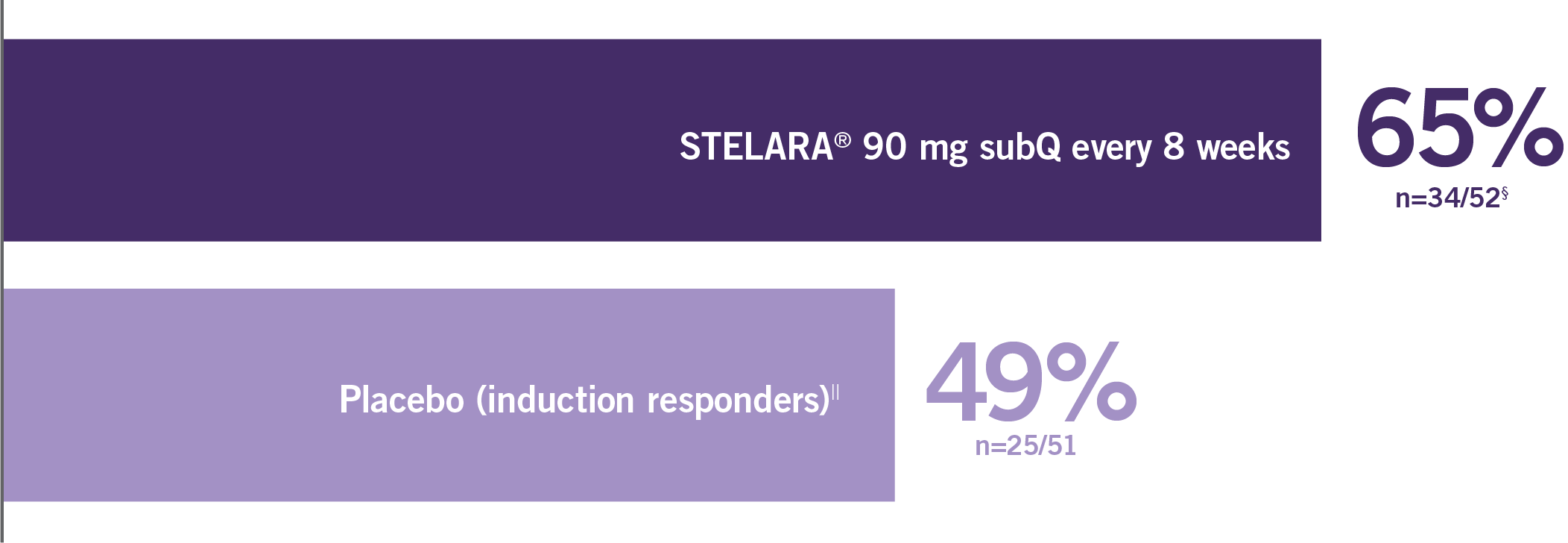
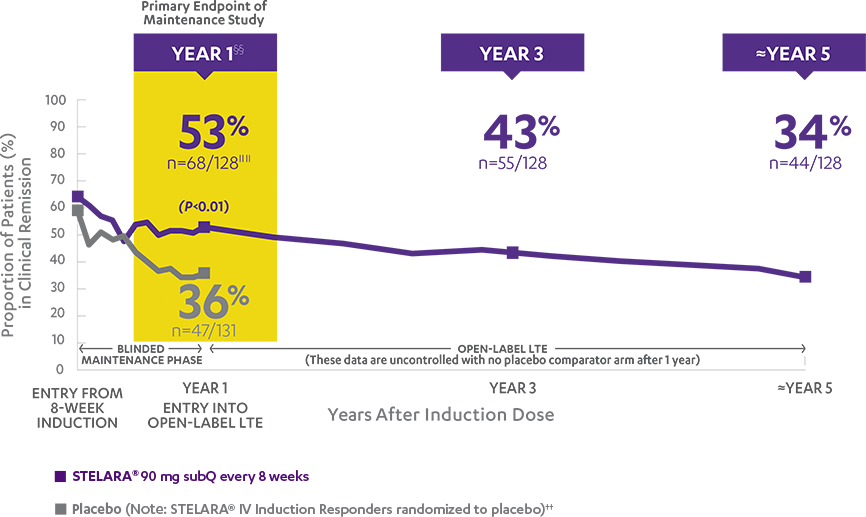
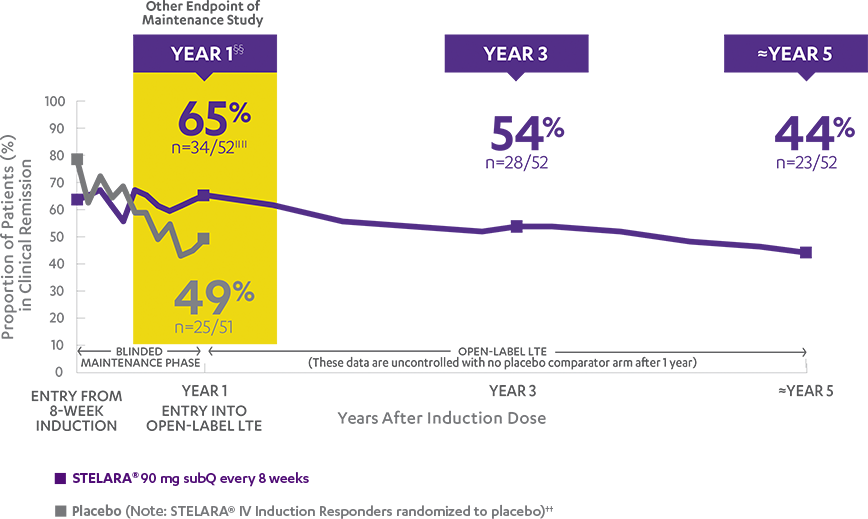
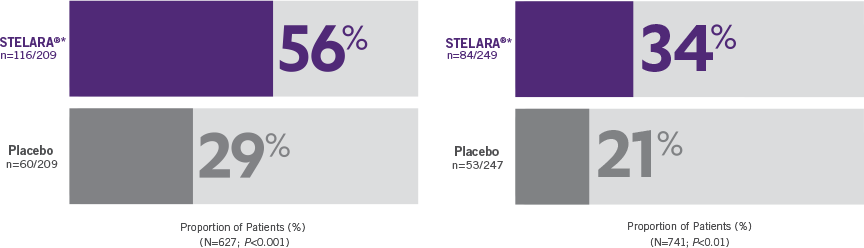
Primary Endpoint
Clinical response at Week 6 (100-point reduction)
Patient population: predominantly TNF blocker-naïve§
(n=116/209)
(n=60/209)
Proportion of Patients (%) (N=627; P<0.001)
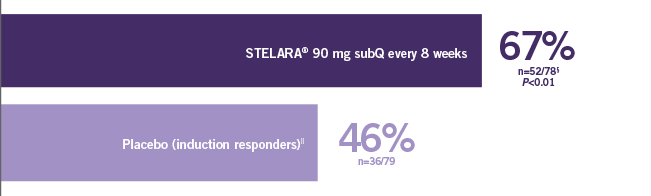
Clinical response at Week 6 (100-point reduction) Patient population: TNF blocker-failure
(n=84/249)
(n=53/247)
Proportion of Patients (%) (N=741; P<0.01)
Clinical response was defined as reduction in CDAI score of ≥100 points or CDAI score of <1501
Significantly greater proportion of predominantly TNF blocker–naïve patients achieved70-point response† at Week 3 (N=627; P<0.001)
(n=106/209)
(n=66/209)
Significant response in predominantlyTNF blocker–naïve patients at Week 6(70-point response†) (N=627; P<0.001)
(n=135/209)
(n=81/209)