IN ACTIVE PSORIATIC ARTHRITISACR RESPONSE RATES OVER TIME1-3
ACR20 Results at Week 24
ACR20 RESPONSE IN BIOLOGIC-NAÏVE PATIENTS THROUGH WEEK 241-3*
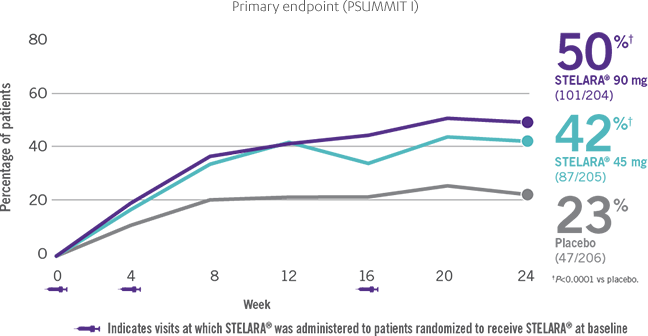
PSUMMIT I: ACR20 response among
biologic-naïve patients through Week 241-3*†

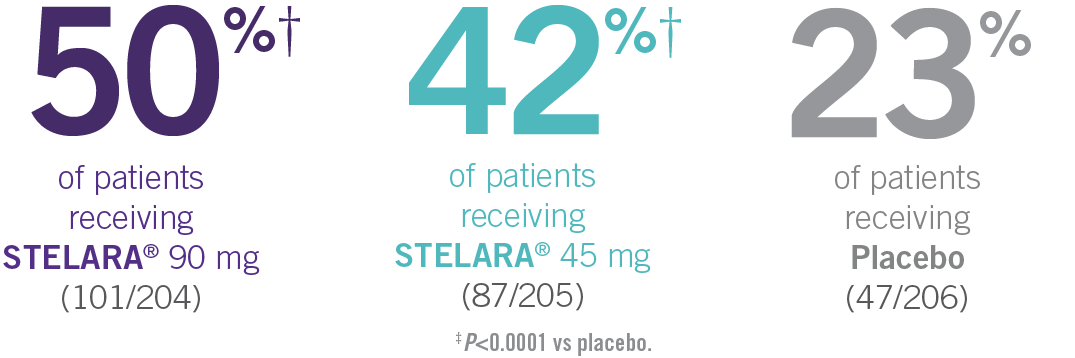
- In PSUMMIT I, ACR20 response was achieved at Week 24 in 42% (87/205) and 50% (101/204) of patients taking STELARA® 45 mg and 90 mg, respectively, vs 23% (47/206) of patients taking placebo (P<0.0001 vs placebo for each dose; primary endpoint)1-3*
- In PSUMMIT I, concomitant MTX use did not appear to influence the efficacy or safety of STELARA®1
In this intent-to-treat analysis, patients were considered treatment failures if they initiated any protocol-prohibited medications, increased the dose of MTX or oral corticosteroids above baseline, or discontinued study agent due to unsatisfactory therapeutic effect for PsA or an adverse event (AE) of worsening PsA or psoriasis. Missing data last-observation-carried-forward rules were applied. The patients who early escaped at Week 16 were considered treatment failures and had Week 16 data carried forward through Week 24.1,3
*Previous exposure to anti-TNF agents was not allowed. Two patients had prior exposure to biologics: 1 to alefacept and 1 to efalizumab.
†In this intent-to-treat analysis, early escape rules were not applied. Efficacy evaluations were based on the subjects’ initial randomized assignment. Subjects who qualified for early escape from the 45-mg group (and therefore received a 90-mg dose starting at Week 16) were counted in the 45-mg group. Concomitant medications were required to remain at stable doses through Week 52.3
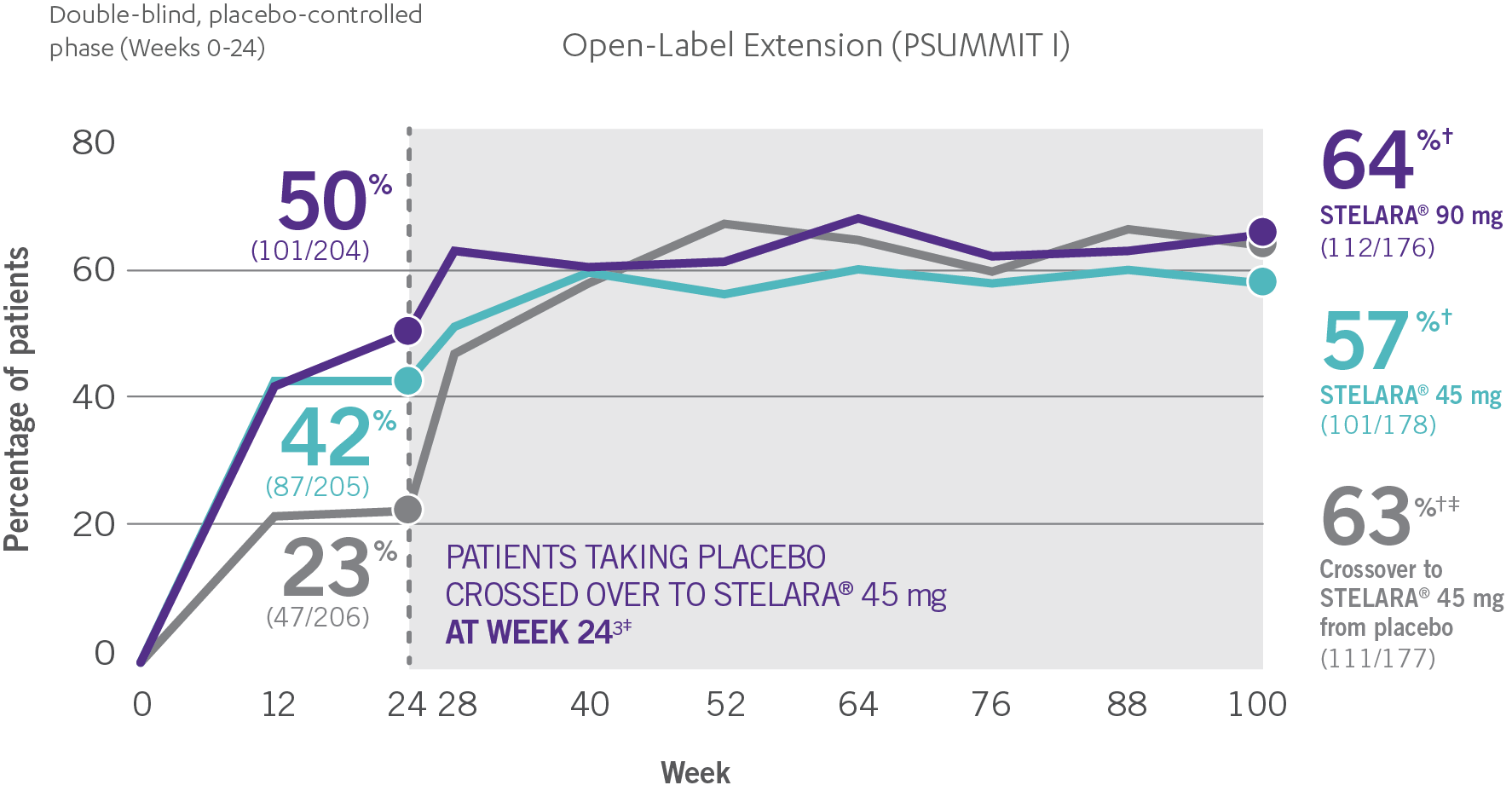
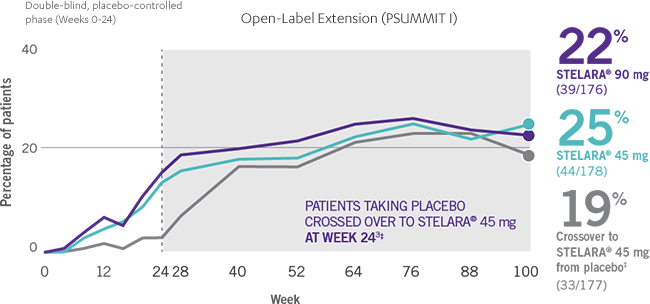
Consistent ACR20 Response Rates Through Week 100
ACR20 RESPONSE IN BIOLOGIC-NAÏVE PATIENTS THROUGH WEEK 1001-4*†
Open-Label Extension (PSUMMIT I)

- The percentage of biologic-naïve STELARA® patients who achieved significant improvement in joint symptoms peaked at Week 28 and reached a plateau at Week 283,4
- Patients were randomized to receive STELARA® 45 mg, STELARA® 90 mg, or placebo as subcutaneous injection at Weeks 0 and 4 and every 12 weeks thereafter. At Week 24, all remaining patients in the placebo group received STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter2
- ACR20 data were collected once every 4 weeks through Week 24, and once every 12 weeks from Week 28 through Week 1003
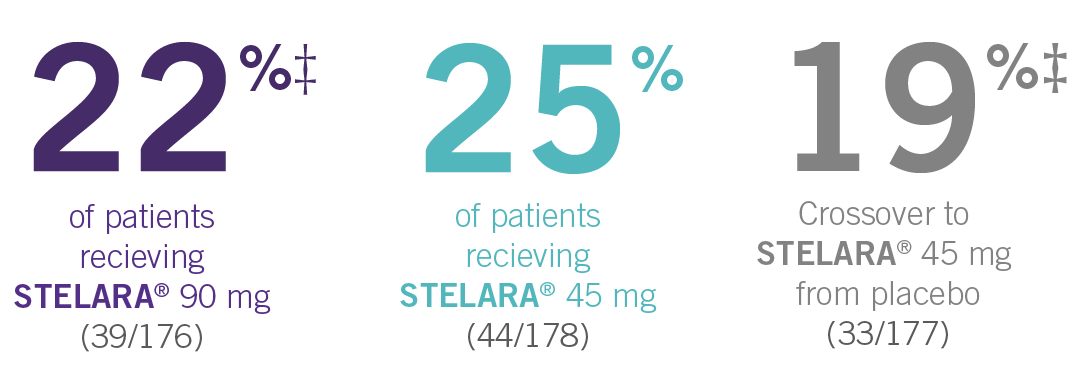
PSUMMIT I was considered an open-label trial after Week 24. At Week 24, all patients in the placebo group who were not eligible for early escape crossed over to STELARA® 45 mg. These patients continued at Week 28 and every 12 weeks thereafter. In this ITT analysis, early escape rules were not applied. At Week 24, the data were unmasked to the sponsor for analysis. Study sites and patients remained masked to treatment assignment until study completion. Patients were considered nonresponders if they discontinued treatment due to an unsatisfactory therapeutic effect, initiated protocol-prohibited medications/therapies, or increased the dose of MTX or oral corticosteroids over baseline prior to Week 52. All placebo patients either entered early escape or crossed over to receive STELARA® 45 mg by Week 24 (n=189).2,3
*PSUMMIT I was considered an open-label trial after Week 24.3
†In this ITT analysis, early escape rules were not applied. Efficacy evaluations were based on the patients’ initial randomized assignment. Patients who qualified for early escape from the 45-mg group (and therefore received a 90-mg dose starting at Week 16) were counted in the 45-mg group. Concomitant medications were required to remain at stable doses through Week 52.3
‡At Week 24, all remaining patients in the placebo group who did not qualify for early escape crossed over to STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter.3
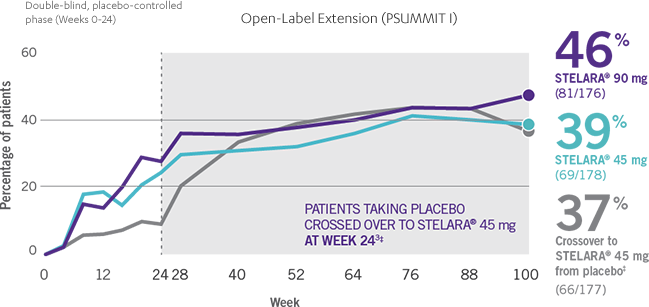
Consistent ACR50 And ACR70 Response Rates Through Week 100
ACR50 RESPONSE RATES OF BIOLOGIC-NAÏVE PATIENTS THROUGH WEEK 1003-4*†
Open-Label Extension (PSUMMIT I)
- Patients in the PSUMMIT I clinical trial were randomized to receive STELARA® 45 mg, STELARA® 90 mg, or placebo as subcutaneous injection at Weeks 0 and 4 and every 12 weeks thereafter. At Week 24, all remaining patients in the placebo group received STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter2,3
- In PSUMMIT I, ACR50 response was achieved at Week 24 in 25% (51/205) and 28% (57/204) of patients taking STELARA® 45 mg and 90 mg, respectively, vs 9% (18/206) of patients taking placebo1,2
- ACR50 data were collected once every 4 weeks through Week 24 and once every 12 weeks from Week 28 through Week 1001

ACR70 RESPONSE RATES OF BIOLOGIC-NAÏVE PATIENTS THROUGH WEEK 1003,4*†
Open-Label Extension (PSUMMIT I)
- Patients in the PSUMMIT I clinical trial were randomized to receive STELARA® 45 mg, STELARA® 90 mg, or placebo as subcutaneous injection at Weeks 0 and 4 and every 12 weeks thereafter. At Week 24, all remaining patients in the placebo group received STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter2,3
- In PSUMMIT I, ACR70 response was achieved at Week 24 in 12% (25/205) and 14% (29/204) of patients taking STELARA® 45 mg and 90 mg, respectively, vs 2% (5/206) of patients taking placebo1,2
- ACR70 data were collected once every 4 weeks through Week 24 and once every 12 weeks from Week 28 through Week 1001
*PSUMMIT I was considered an open-label trial after Week 24.3
†In this ITT analysis, early escape rules were not applied. Efficacy evaluations were based on the patients’ initial randomized assignment. Patients who qualified for early escape from the 45-mg group (and therefore received a 90-mg dose starting at Week 16) were counted in the 45-mg group. Concomitant medications were required to remain at stable doses through Week 52.3
‡At Week 24, all remaining patients in the placebo group who did not qualify for early escape crossed over to STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter.3
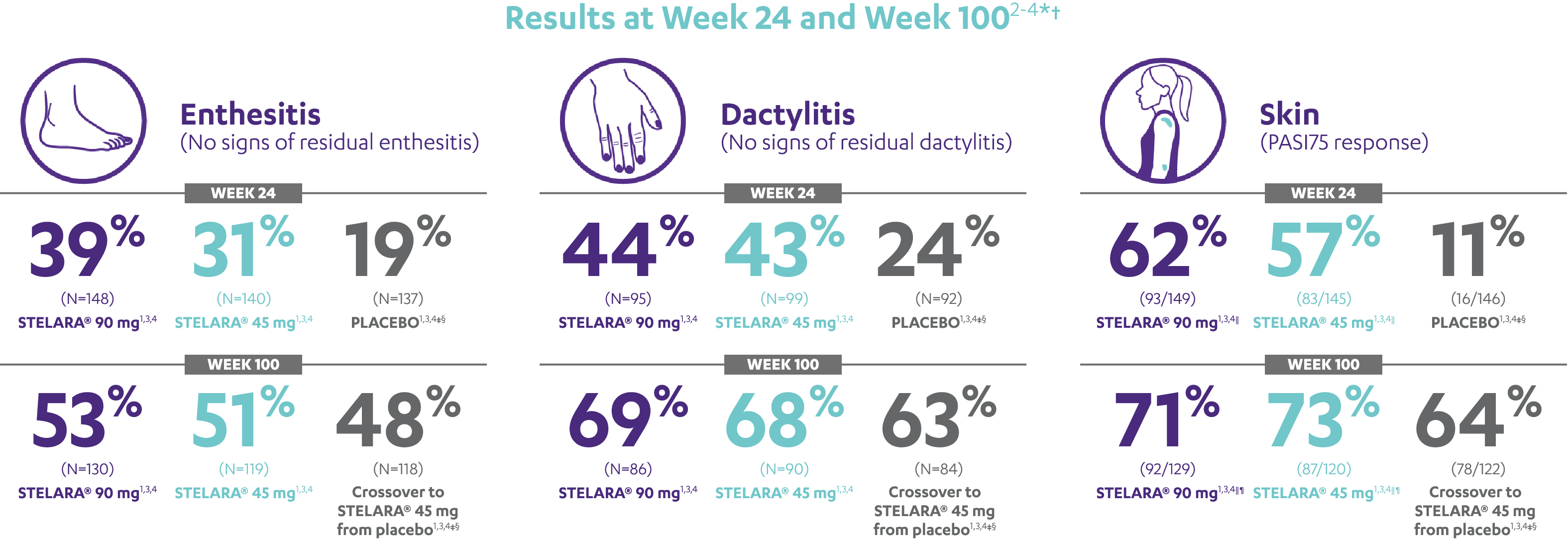
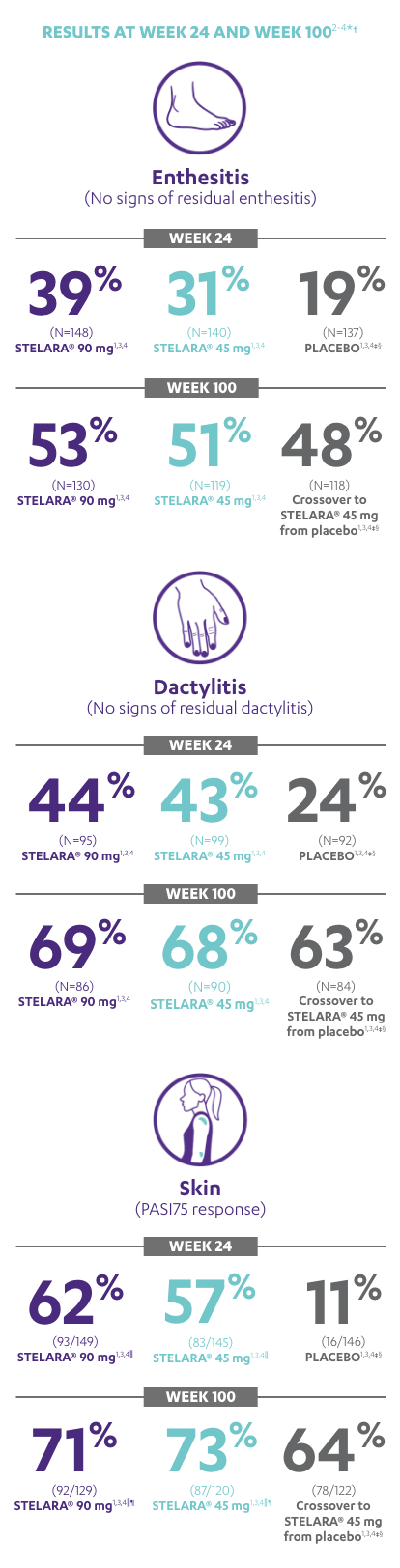
IN ACTIVE PSORIATIC ARTHRITISIMPROVEMENT SEEN ACROSS DOMAINS OF PsA
RESULTS AT WEEK 24 AND WEEK 1002-4*†
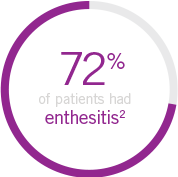
Note: Enthesitis was measured only in patients with enthesitis at baseline. The MASES index was modified for PsA to include 2 additional sites (left and right insertion of the plantar fascia) so that a total of 15 enthesitis sites were evaluated. Enthesitis scores range from 0 to 15, with diagnosis if the score is >0.
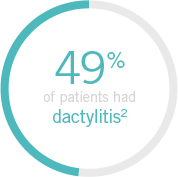
Note: Dactylitis was measured only in patients with dactylitis in ≥1 digit at baseline. Dactylitis was assessed in the 20 digits of the hands and feet on a scale of 0 to 3 (0=no dactylitis, 3=severe dactylitis) for a total of 60 points, with diagnosis if the score was >0.
*PSUMMIT I was considered an open-label trial after Week 24.3
†In this ITT analysis, early escape rules were not applied. Efficacy evaluations were based on the patients’ initial randomized assignment. Patients who qualified for early escape from the 45-mg group (and therefore received a 90-mg injection starting at Week 16) were counted in the 45-mg group. Concomitant medications were required to remain at stable doses through Week 52.3
‡At Week 24, all remaining patients in the placebo group who did not qualify for early escape, crossed over to STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter.3
§Patients who did not receive an injection of STELARA® after crossing over to STELARA® 45 mg from the placebo group were excluded from the enthesitis/dactylitis analysis.3
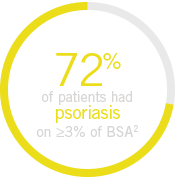
||In patients with ≥3% body surface area (BSA) affected by plaque psoriasis at baseline.
¶Previous exposure to anti-TNF agents was not allowed. Two patients had prior exposure to biologics: 1 to alefacept and 1 to efalizumab.
References: 1. STELARA [package insert]. Horsham, PA: Janssen Biotech, Inc; 2016. 2. McInnes IB, Kavanaugh A, Gottlieb AB, et al; for the PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. 3. Data on file. Janssen Biotech, Inc. 4. Kavanaugh A, Puig L, Gottlieb AB, et al; on behalf of the PSUMMIT 1 Study Group. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res (Hoboken). 2015;67(12):1739-1749.
Trial population

General population
up to 24% of patients develop joint symptoms before any other symptoms5*
*In North America. The MAPP survey is a multinational, large-scale probability survey based on psoriasis and PsA national samplings of households in the United States, Canada, France, Germany, Italy, Spain, and the United Kingdom.
Trial population
General population
up to 78% of patients present with enthesitis6
Trial population
General population
up to 48% of patients present with dactylitis7
Trial population
General population
up to 80% of patients present with psoriasis
before the onset of PsA8
Trial population
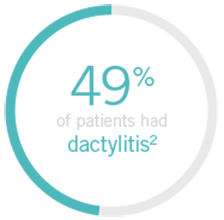
General population1
Up to 48% of patients present with dactylitis7
Trial population
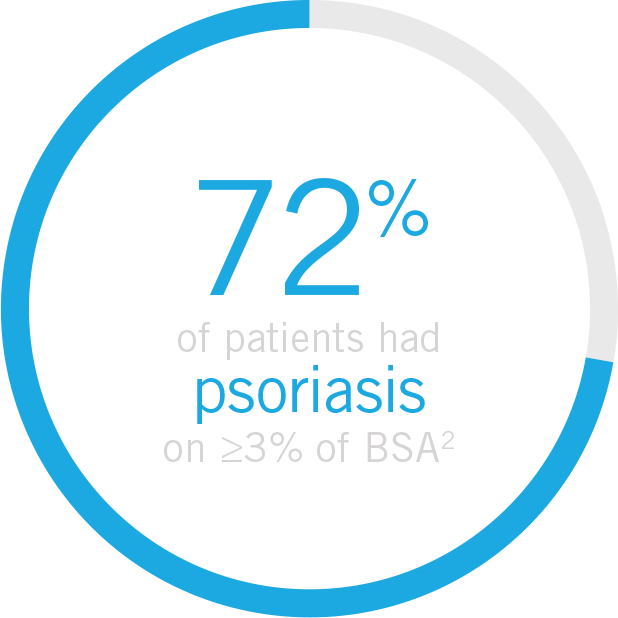
General population
Up to 80% of patients present with psoriasis before the onset of PsA8