IN ACTIVE PSORIATIC ARTHRITIS
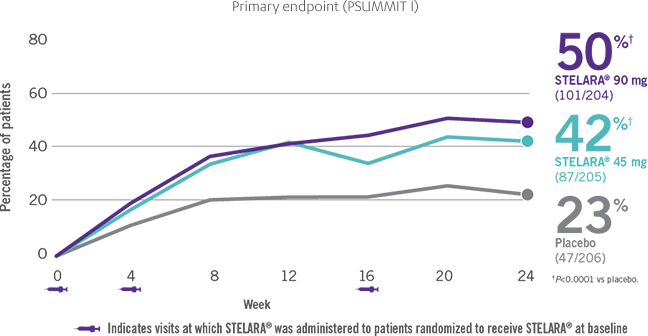
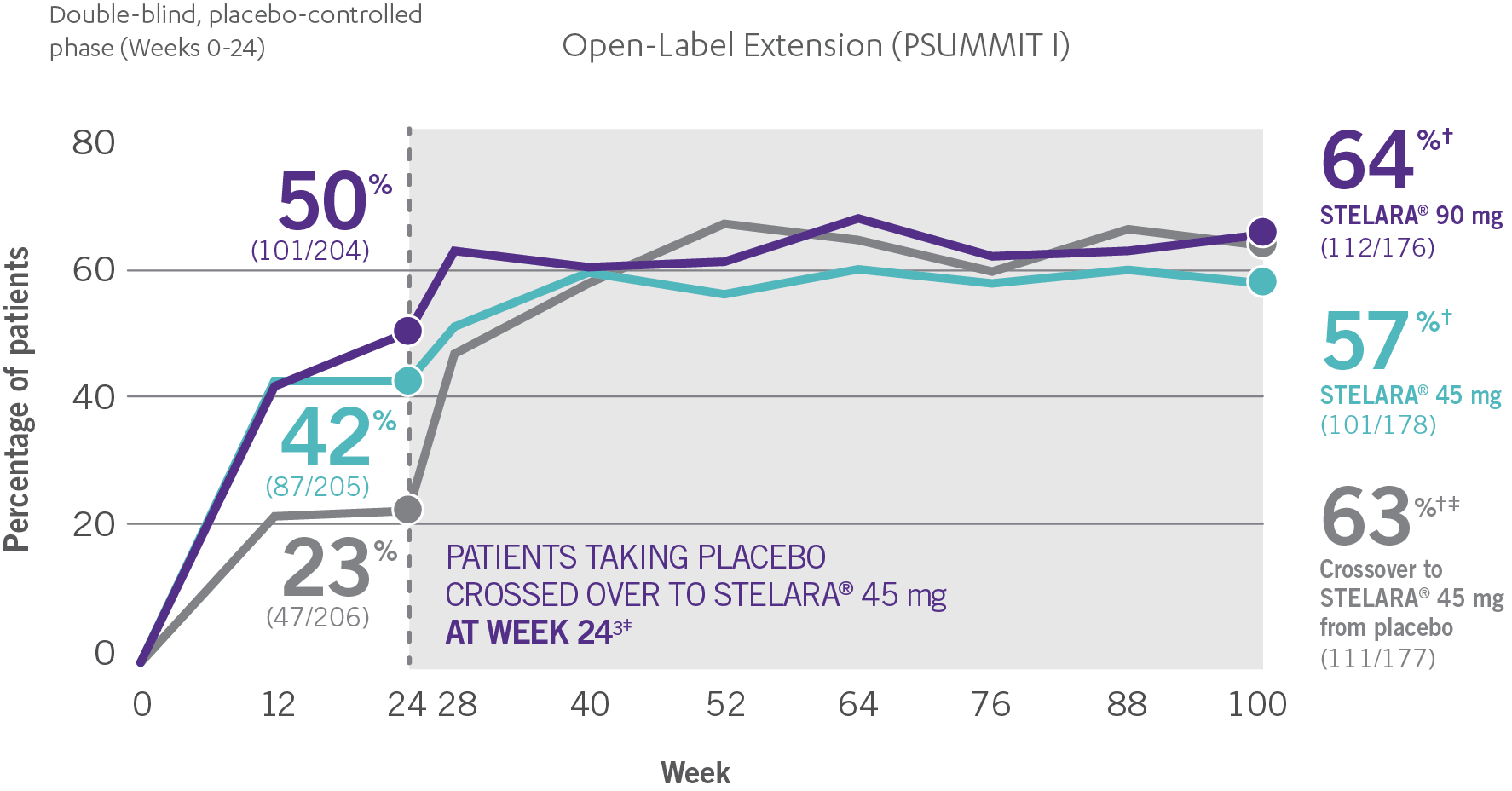
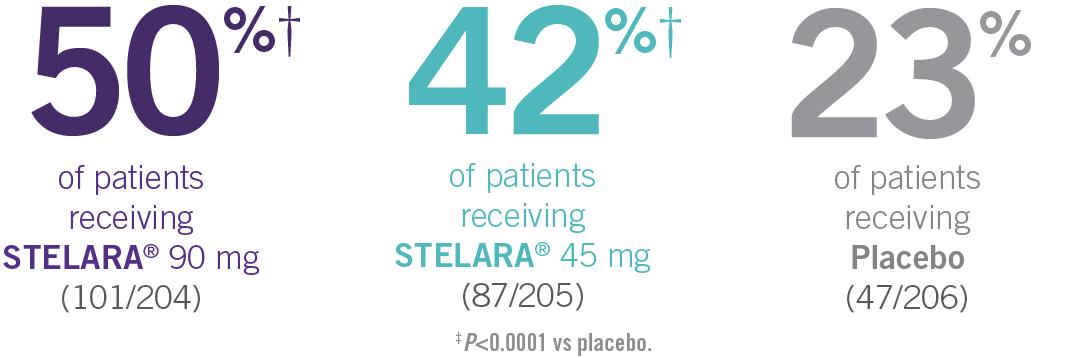
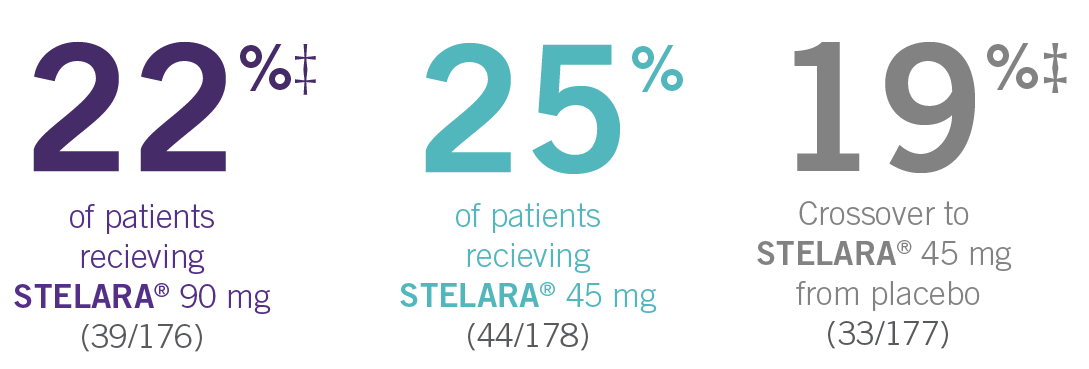
ACR RESPONSE RATES OVER TIME1-3
IN ACTIVE PSORIATIC ARTHRITIS
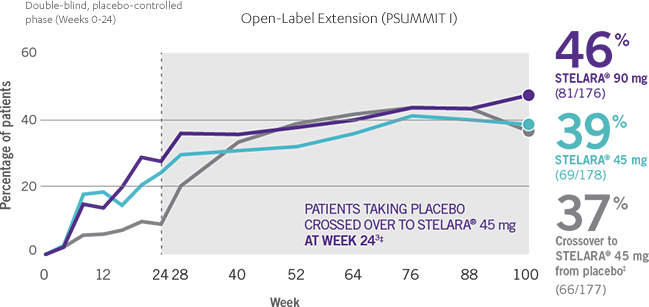
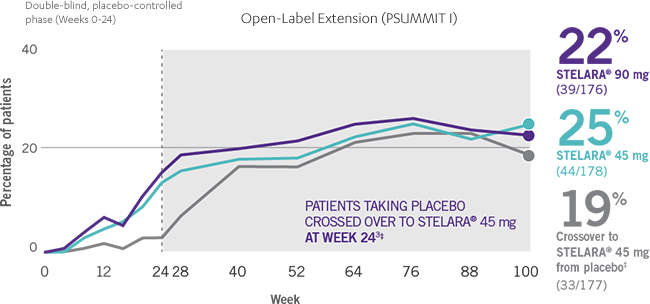
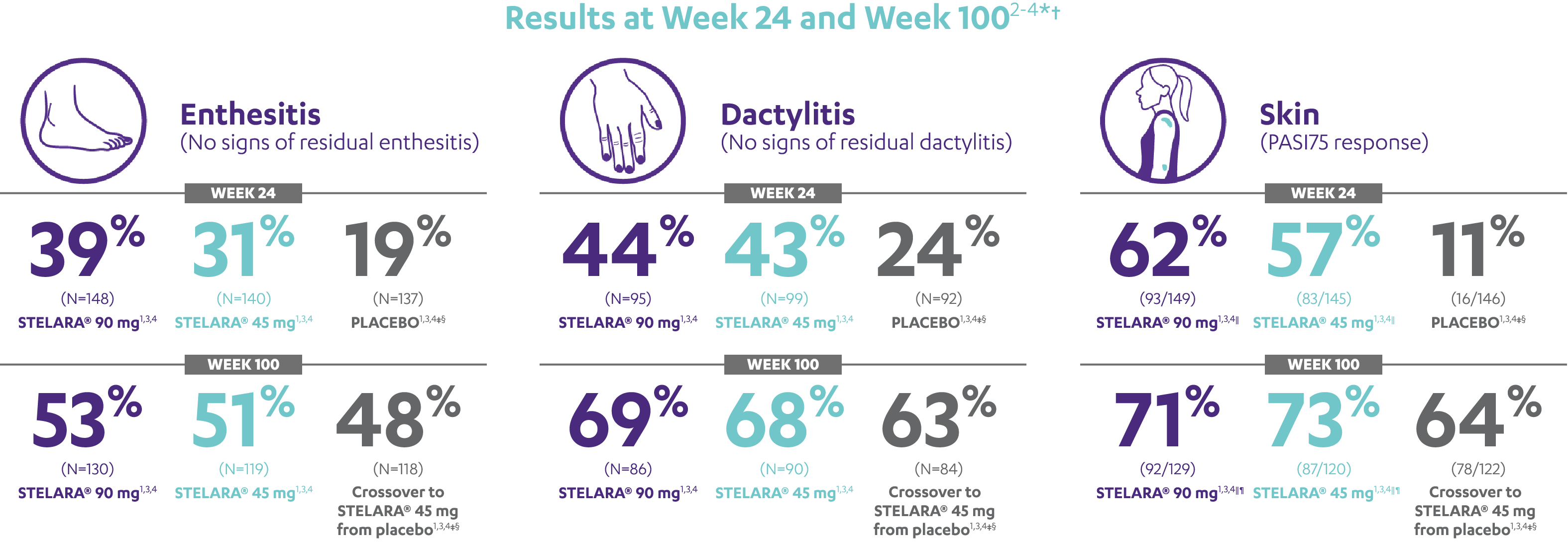
IMPROVEMENT SEEN ACROSS DOMAINS OF PsA
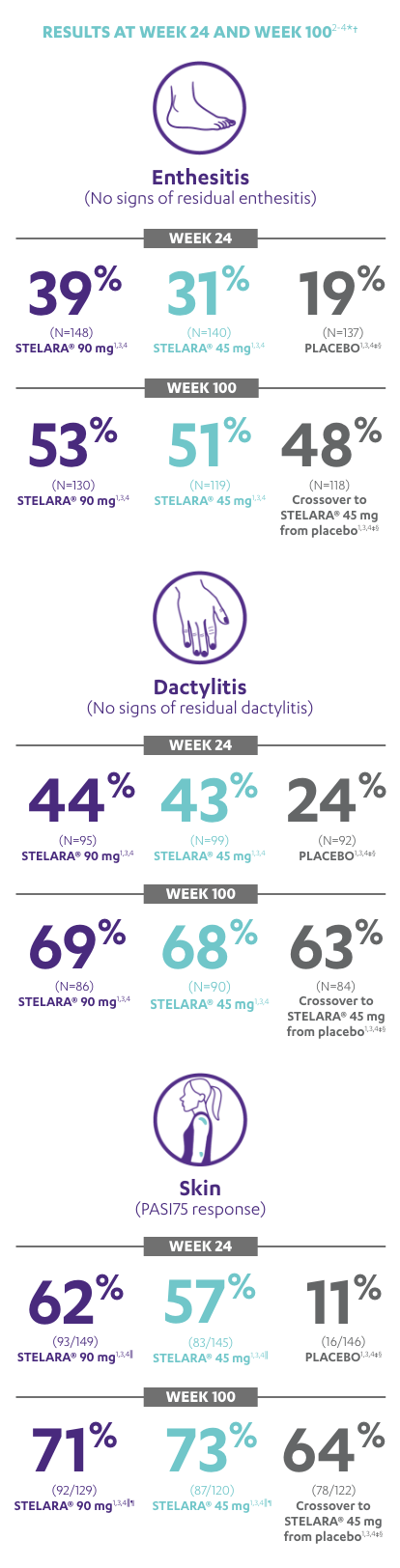
RESULTS AT WEEK 24 AND WEEK 1002-4*†
Note: Enthesitis was measured only in patients with enthesitis at baseline. The MASES index was modified for PsA to include 2 additional sites (left and right insertion of the plantar fascia) so that a total of 15 enthesitis sites were evaluated. Enthesitis scores range from 0 to 15, with diagnosis if the score is >0.
Note: Dactylitis was measured only in patients with dactylitis in ≥1 digit at baseline. Dactylitis was assessed in the 20 digits of the hands and feet on a scale of 0 to 3 (0=no dactylitis, 3=severe dactylitis) for a total of 60 points, with diagnosis if the score was >0.
*PSUMMIT I was considered an open-label trial after Week 24.3
†In this ITT analysis, early escape rules were not applied. Efficacy evaluations were based on the patients’ initial randomized assignment. Patients who qualified for early escape from the 45-mg group (and therefore received a 90-mg injection starting at Week 16) were counted in the 45-mg group. Concomitant medications were required to remain at stable doses through Week 52.3
‡At Week 24, all remaining patients in the placebo group who did not qualify for early escape, crossed over to STELARA® 45 mg, which they continued at Week 28 and every 12 weeks thereafter.3
§Patients who did not receive an injection of STELARA® after crossing over to STELARA® 45 mg from the placebo group were excluded from the enthesitis/dactylitis analysis.3
||In patients with ≥3% body surface area (BSA) affected by plaque psoriasis at baseline.
¶Previous exposure to anti-TNF agents was not allowed. Two patients had prior exposure to biologics: 1 to alefacept and 1 to efalizumab.
References: 1. STELARA [package insert]. Horsham, PA: Janssen Biotech, Inc; 2016. 2. McInnes IB, Kavanaugh A, Gottlieb AB, et al; for the PSUMMIT 1 Study Group. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382(9894):780-789. 3. Data on file. Janssen Biotech, Inc. 4. Kavanaugh A, Puig L, Gottlieb AB, et al; on behalf of the PSUMMIT 1 Study Group. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res (Hoboken). 2015;67(12):1739-1749.