IN ACTIVE PSORIATIC ARTHRITIS
PROVEN SAFETY PROFILE
PHASE 3: POOLED SAFETY DATA: PSUMMIT I AND PSUMMIT II AT WEEK 16
(PLACEBO-CONTROLLED PERIOD)1
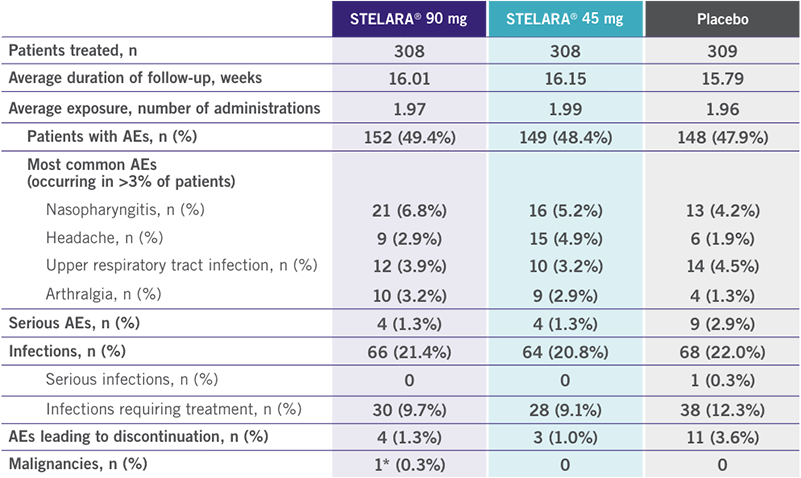


- Through Week 16, no cases of tuberculosis (TB) or opportunistic infections were reported1
- Through Week 24, injection-site reactions occurred in 14 (1.5%) patients taking placebo, 6 (1.5%) patients taking STELARA® 45 mg, and 8 (2.2%) patients taking STELARA® 90 mg1
*Squamous cell carcinoma in situ in an area of cleared psoriasis.1

IN ACTIVE PSORIATIC ARTHRITIS
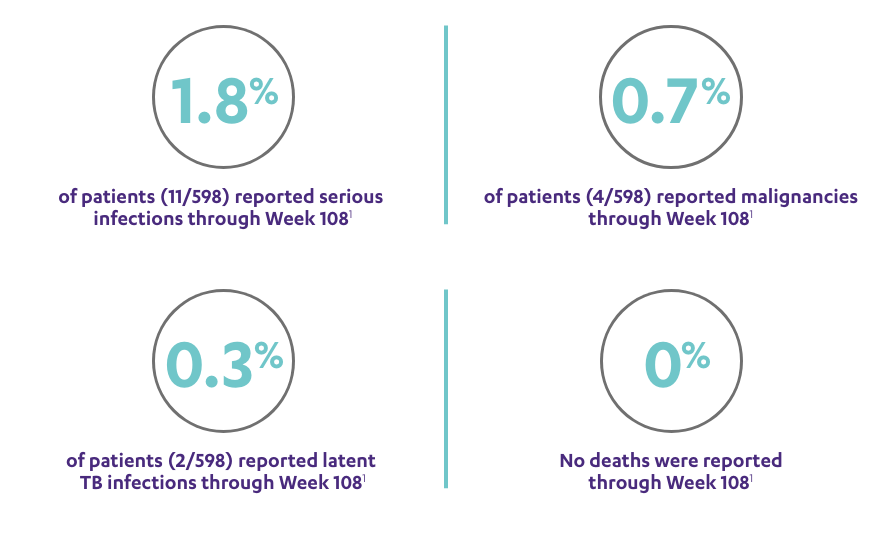
CONSISTENT SAFETY THROUGH WEEK 108
SAFETY DATA THROUGH WEEK 1081,3*
Open-Label Extension (PSUMMIT I)
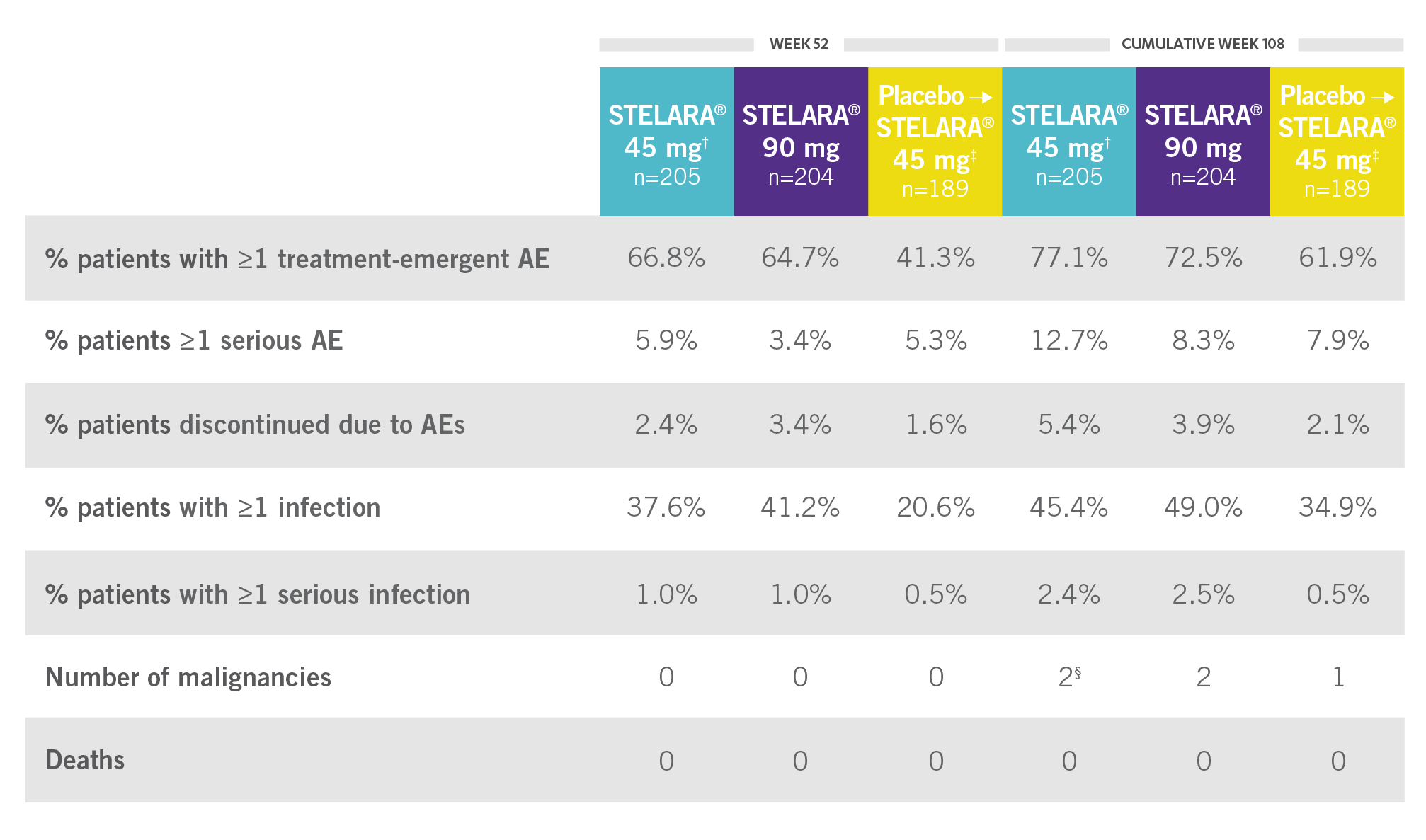
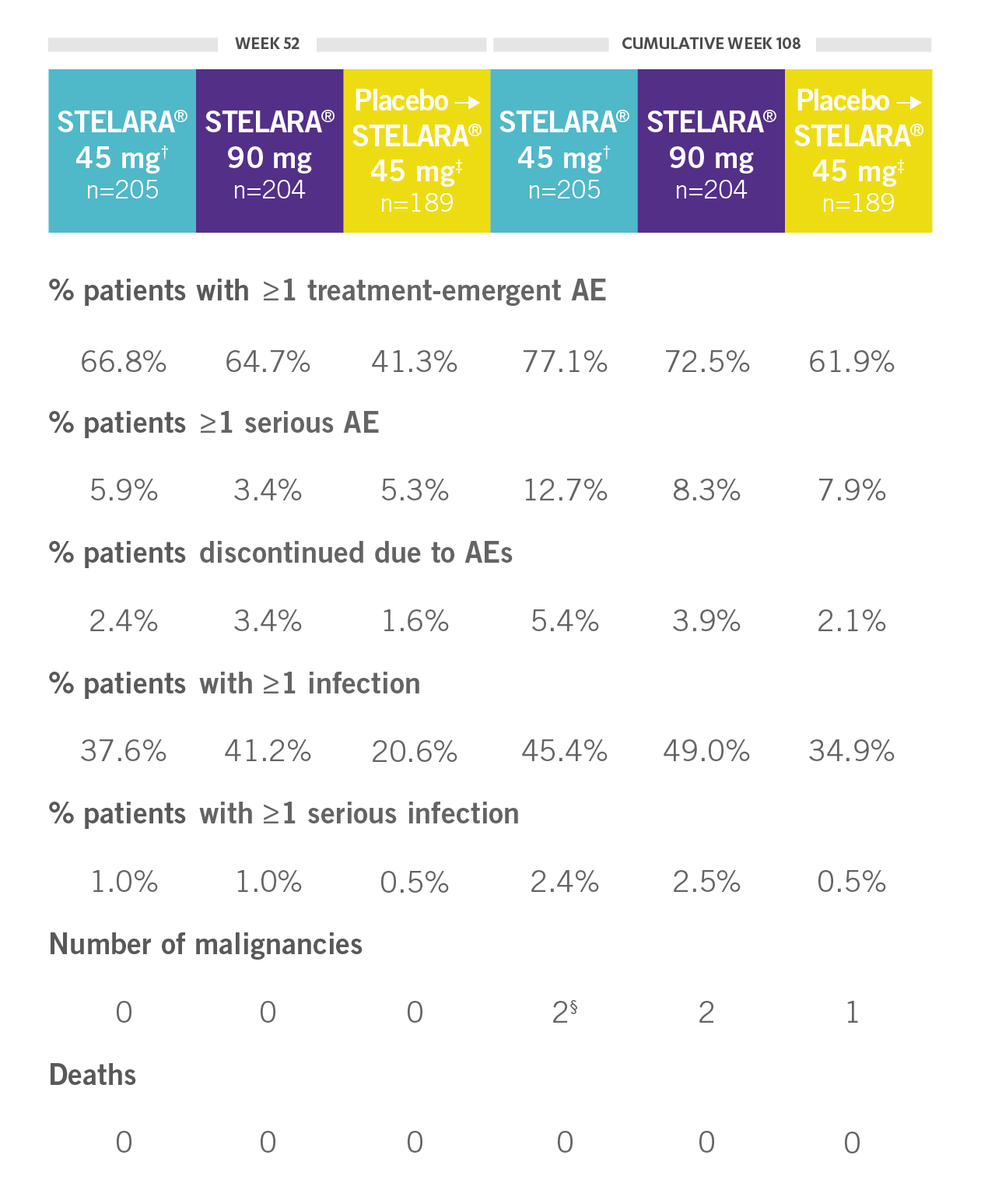

RATE OF SELECTED TREATMENT-EMERGENT AEs THROUGH WEEK 108 (PSUMMIT I OPEN-LABEL EXTENSION)*‖
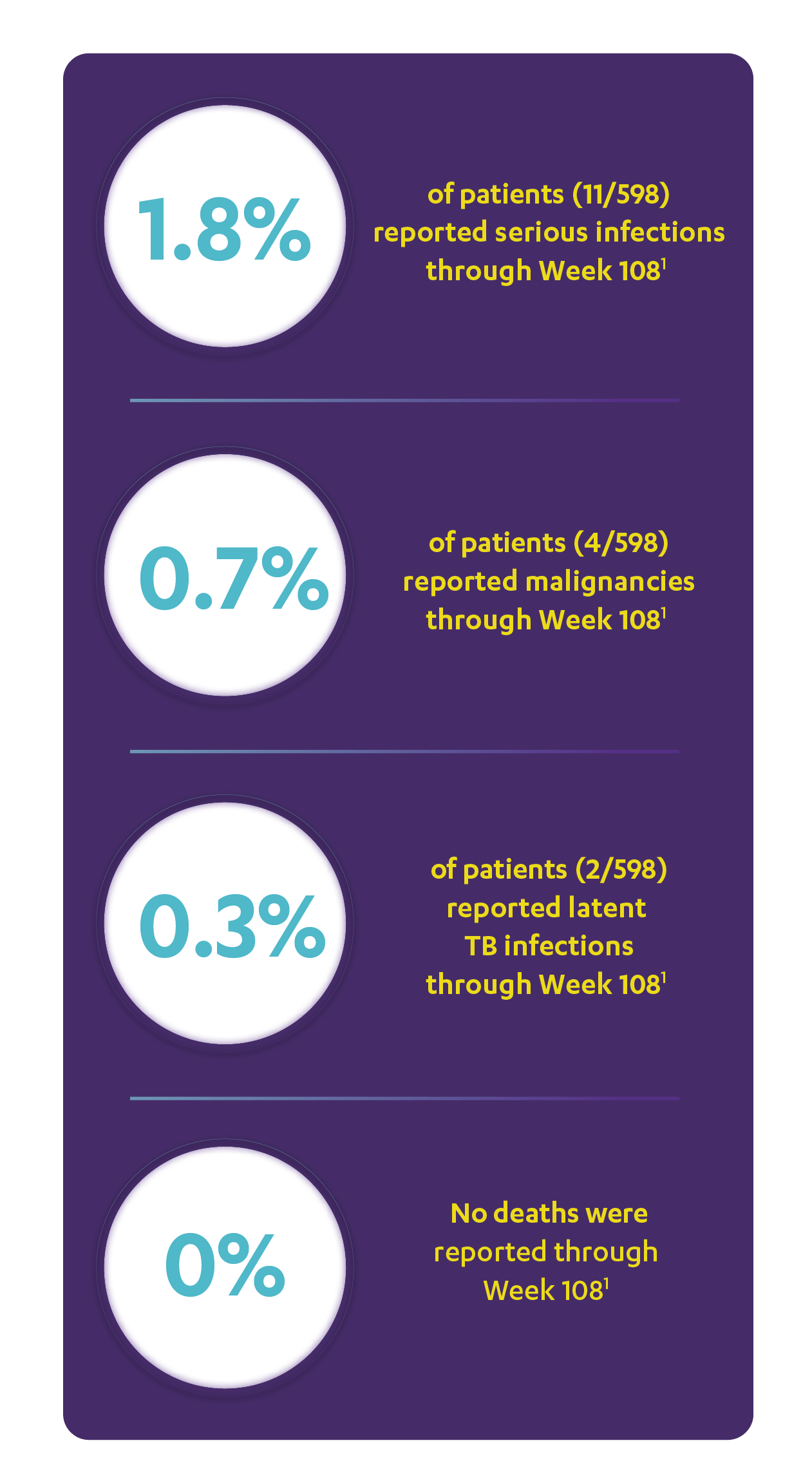

*PSUMMIT I was considered an open-label trial after Week 24.2
†Combined 45-mg group: includes those randomized to 45 mg and those switched from 45 mg to 90 mg.2
‡Includes all patients randomized to the placebo group, including those who early escaped at Week 16 to receive STELARA® 45 mg.
§One of the malignancies was from the placebo crossover to 45-mg group.2
‖Includes patients who received STELARA® 45 mg and those who received STELARA® 90 mg.2
References: 1. Data on file. Janssen Biotech, Inc. 2. STELARA® [Prescribing Information]. Horsham, PA: Janssen Pharmaceuticals, Inc; 2019. 3. Kavanaugh A, Puig L, Gottlieb AB, et al; on behalf of the PSUMMIT 1 Study Group. Maintenance of clinical efficacy and radiographic benefit through two years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res (Hoboken). 2015;67(12):1739-1749.