In adults with moderate to severe plaque psoriasisfor adults with moderate to severe plaque psoriasis
Uncover Response Rates Through Week 121,2
PHOENIX 1—PASI 75 response at Week 12 (PRIMARY ENDPOINT)1,2*†
 Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.
Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.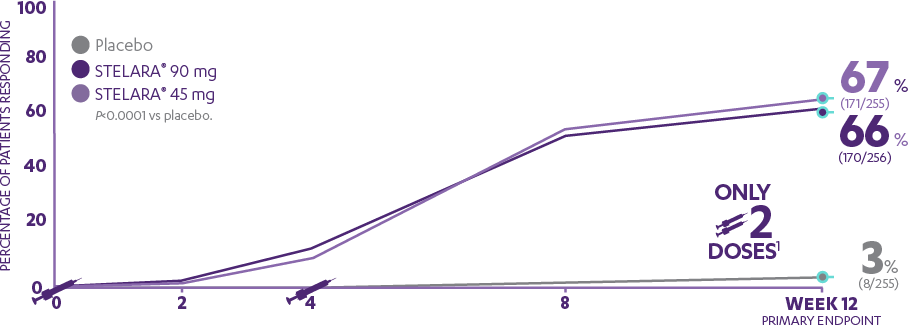
IN PHOENIX 1:
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by nearly 6 of 10 patients in the 45-mg and 90-mg groups (59% [151/255] and 61% [156/256], respectively) compared with 4% (10/255) in the placebo group.1,2
IN PHOENIX 2:
The primary endpoint was PASI 75 at Week 12 (45 mg: 67% [273/409]; 90 mg: 76% [311/411]; placebo: 4% [15/410]; P<0.0001 vs placebo for each dose).1,3
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
†Patients who discontinued study agent because of lack of efficacy or an adverse event of psoriasis worsening, or who started a protocol-prohibited psoriasis treatment were considered nonresponders (binary endpoints). Other patients with missing data were considered nonresponders for binary endpoints and nonresponder imputation (NRI) was applied.
PASI=Psoriasis Area and Severity Index; PGA=Physician's Global Assessment; PGA 0/1=Proportion of patients who achieved a PGA score of cleared (0) or minimal (1) using a 5-point scale: cleared (0), minimal (1), mild (2), moderate (3), marked (4), and severe (5).
*PASI 75 responder was defined as patients with greater than or equal to 75% improvement in PASI from baseline.
PHOENIX 1—PASI 90 response at Week 12 (PRESPECIFIED OTHER SECONDARY ENDPOINT)1,2*†
 Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.
Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.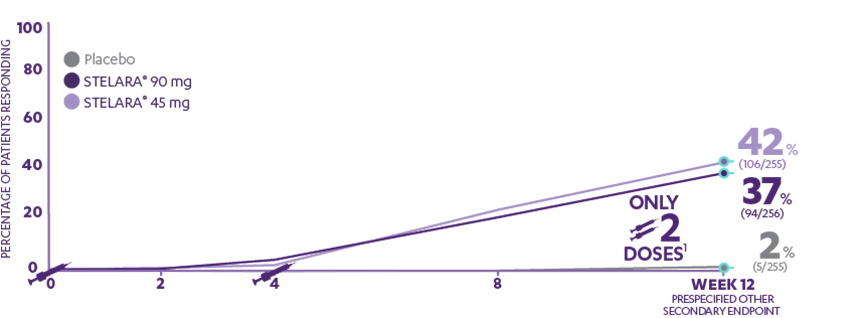
IN PHOENIX 2:
At Week 12, PASI 90 was 42% (173/409) and 51% (209/411) in patients taking STELARA® 45 mg and 90 mg, respectively, vs 1% (3/410) of patients taking placebo.1,3
*PASI 90 is a prespecified analysis that was not adjusted for multiplicity; statistical significance has not been established.
†PASI 90 responder was defined as patients with greater than or equal to 90% improvement in PASI from baseline.
PHOENIX 1—PASI 75 response at Week 12 (PRIMARY ENDPOINT)1,2*†
66%
of patients receiving STELARA® 90 mg (170/256) achieved PASI 75 response (P<0.0001 vs placebo).
67%
of patients receiving STELARA® 45 mg (171/255) achieved PASI 75 response (P<0.0001 vs placebo).
vs 3% of patients receiving placebo (8/255)
AFTER Only 2 doses1
IN PHOENIX 1:
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by nearly 6 of 10 patients in the 45-mg and 90-mg groups (59% [151/255] and 61% [156/256], respectively) compared with 4% (10/255) in the placebo group.1,2
IN PHOENIX 2:
The primary endpoint was PASI 75 at Week 12 (45 mg: 67% [273/409]; 90 mg: 76% [311/411]; placebo: 4% [15/410]; P<0.0001 vs placebo for each dose).1,3
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
†Patients who discontinued study agent because of lack of efficacy or an adverse event of psoriasis worsening, or who started a protocol-prohibited psoriasis treatment were considered nonresponders (binary endpoints). Other patients with missing data were considered nonresponders for binary endpoints and nonresponder imputation (NRI) was applied.
PASI=Psoriasis Area and Severity Index; PGA=Physician's Global Assessment; PGA 0/1=Proportion of patients who achieved a PGA score of cleared (0) or minimal (1) using a 5-point scale: cleared (0), minimal (1), mild (2), moderate (3), marked (4), and severe (5).
*PASI 75 responder was defined as patients with greater than or equal to 75% improvement in PASI from baseline.
PHOENIX 1—PASI 90 response at Week 12 (PRESPECIFIED OTHER SECONDARY ENDPOINT)1,2*†
37%
of patients receiving STELARA®
90 mg (94/256) achieved PASI 90 response
90 mg (94/256) achieved PASI 90 response
42%
of patients receiving STELARA® 45 mg (106/255) achieved PASI 90 response
vs 2% of patients receiving placebo (5/255)
AFTER Only 2 doses1
IN PHOENIX 2:
At Week 12, PASI 90 was 42% (173/409) and 51% (209/411) in patients taking STELARA® 45 mg and 90 mg, respectively, vs 1% (3/410) of patients taking placebo.1,3
*PASI 90 is a prespecified analysis that was not adjusted for multiplicity; statistical significance has not been established.
†PASI 90 responder was defined as patients with greater than or equal to 90% improvement in PASI from baseline.
In adults with moderate to severe plaque psoriasis
Discover Consistent Response Rates Over Time
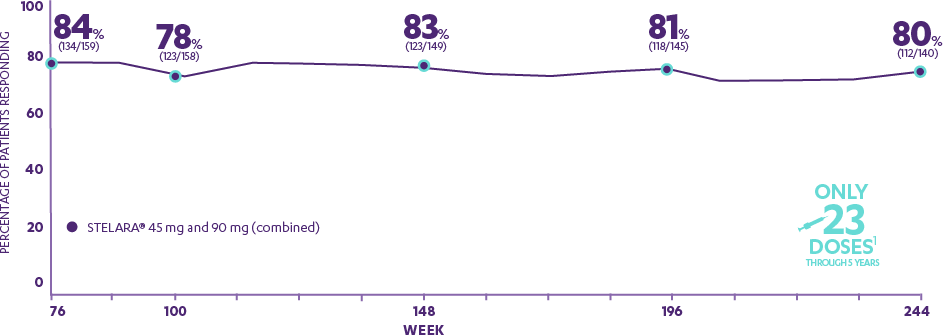
PHOENIX 1—PRESPECIFIED ENDPOINT: PASI 75 response at Week 76 and in an open-label extension through 5 years1,4,5*†‡§‖
Patients who were rerandomized to continue every-12-week dosing after responding to STELARA® at Weeks 28 and 40
*PASI 75 at Weeks 76, 160, and 244 were prespecified analyses that were not adjusted for multiplicity; statistical significance has not been established.
†The same patients may not have responded at each time point.
‡Patients who discontinued study treatment due to unsatisfactory therapeutic effect or an AE of worsening psoriasis, or who started a protocol-prohibited medication or therapy during that study that could improve psoriasis were considered treatment failures. Weeks 40-244: treatment failure rules (TFR) were applied.
§Open label period began at Week 52. Treatment was unblinded at Week 76 and TFR was relaxed to allow for the use of concomitant topical medications except for high-potency corticosteroids.
‖Initial responders who were rerandomized at Week 40 to withdraw from ustekinumab and reinitiate treatment upon loss of response are not included in this prespecified analysis.
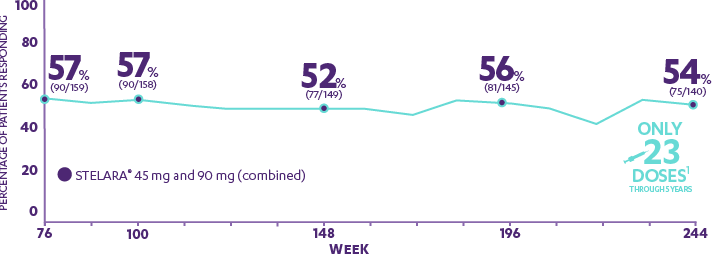
PHOENIX 1—PRESPECIFIED ENDPOINT: PASI 90 response at Week 76 and in an open-label extension through 5 years4,5*†‡§‖
Patients who were rerandomized to every-12-week dosing after responding to STELARA® at Weeks 28 and 40
†PASI 90 at Weeks 76, 160, and 244 were prespecified analyses that were not adjusted for multiplicity; statistical significance has not been established.
‡The same patients may not have responded at each time point.
§Patients who discontinued study treatment due to unsatisfactory therapeutic effect or an AE of worsening psoriasis, or who started a protocol-prohibited medication or therapy during that study that could improve psoriasis were considered treatment failures. Weeks 40-244: treatment failure rules (TFR) were applied.
‖Open label period began at Week 52. Treatment was unblinded at Week 76 and TFR was relaxed to allow for the use of concomitant topical medications except for high-potency corticosteroids.
¶Initial responders who were rerandomized at Week 40 to withdraw from ustekinumab and reinitiate treatment upon loss of response are not included in this prespecified analysis.
PHOENIX 1—PRESPECIFIED ENDPOINT: PASI 75 response at Week 76 and in an open-label extension through 5 years1,4,5*†‡§‖
Patients who were rerandomized to continue every-12-week dosing after responding to STELARA® at Weeks 28 and 40
- 84%
- 78%
- 83%
- 81%
- 80%
- WEEK 76
- WEEK 100
- WEEK 148
- WEEK 196
- WEEK 244
Only 23 Doses1
Through 5 Years
Through 5 Years
*PASI 75 at Weeks 76, 160, and 244 were prespecified analyses that were not adjusted for multiplicity; statistical significance has not been established.
†The same patients may not have responded at each time point.
‡Patients who discontinued study treatment due to unsatisfactory therapeutic effect or an AE of worsening psoriasis, or who started a protocol-prohibited medication or therapy during that study that could improve psoriasis were considered treatment failures. Weeks 40-244: treatment failure rules (TFR) were applied.
§Open label period began at Week 52. Treatment was unblinded at Week 76 and TFR was relaxed to allow for the use of concomitant topical medications except for high-potency corticosteroids.
‖Initial responders who were rerandomized at Week 40 to withdraw from ustekinumab and reinitiate treatment upon loss of response are not included in this prespecified analysis.
PHOENIX 1—PRESPECIFIED ENDPOINT: PASI 90 response at Week 76 and in an open-label extension through 5 years4,5*†‡§‖
Patients who were rerandomized to every-12-week dosing after responding to STELARA® at Weeks 28 and 40
- 57%
- 57%
- 52%
- 56%
- 54%
- WEEK 76
- WEEK 100
- WEEK 148
- WEEK 196
- WEEK 244
Only 23 Doses1
Through 5 Years
Through 5 Years
†PASI 90 at Weeks 76, 160, and 244 were prespecified analyses that were not adjusted for multiplicity; statistical significance has not been established.
‡The same patients may not have responded at each time point.
§Patients who discontinued study treatment due to unsatisfactory therapeutic effect or an AE of worsening psoriasis, or who started a protocol-prohibited medication or therapy during that study that could improve psoriasis were considered treatment failures. Weeks 40-244: treatment failure rules (TFR) were applied.
‖Open label period began at Week 52. Treatment was unblinded at Week 76 and TFR was relaxed to allow for the use of concomitant topical medications except for high-potency corticosteroids.
¶Initial responders who were rerandomized at Week 40 to withdraw from ustekinumab and reinitiate treatment upon loss of response are not included in this prespecified analysis.
References: 1. STELARA® (ustekinumab) [package insert]. Horsham, PA: Janssen Biotech, Inc. 2. Leonardi CL, Kimball AB, Papp KA, et al; for the PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665-1674. 3. Papp KA, Langley RG, Lebwohl M, et al; for the PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675-1684. 4. Data on file. Janssen Biotech, Inc. 5. Kimball AB, Papp KA, Wasfi Y, et al; on behalf of the PHOENIX 1 investigators. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27(12):1535-1545.
cp-168045v4 11/25
cp-168045v4 11/25