IN ADULTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS
STELARA® Safety Profile
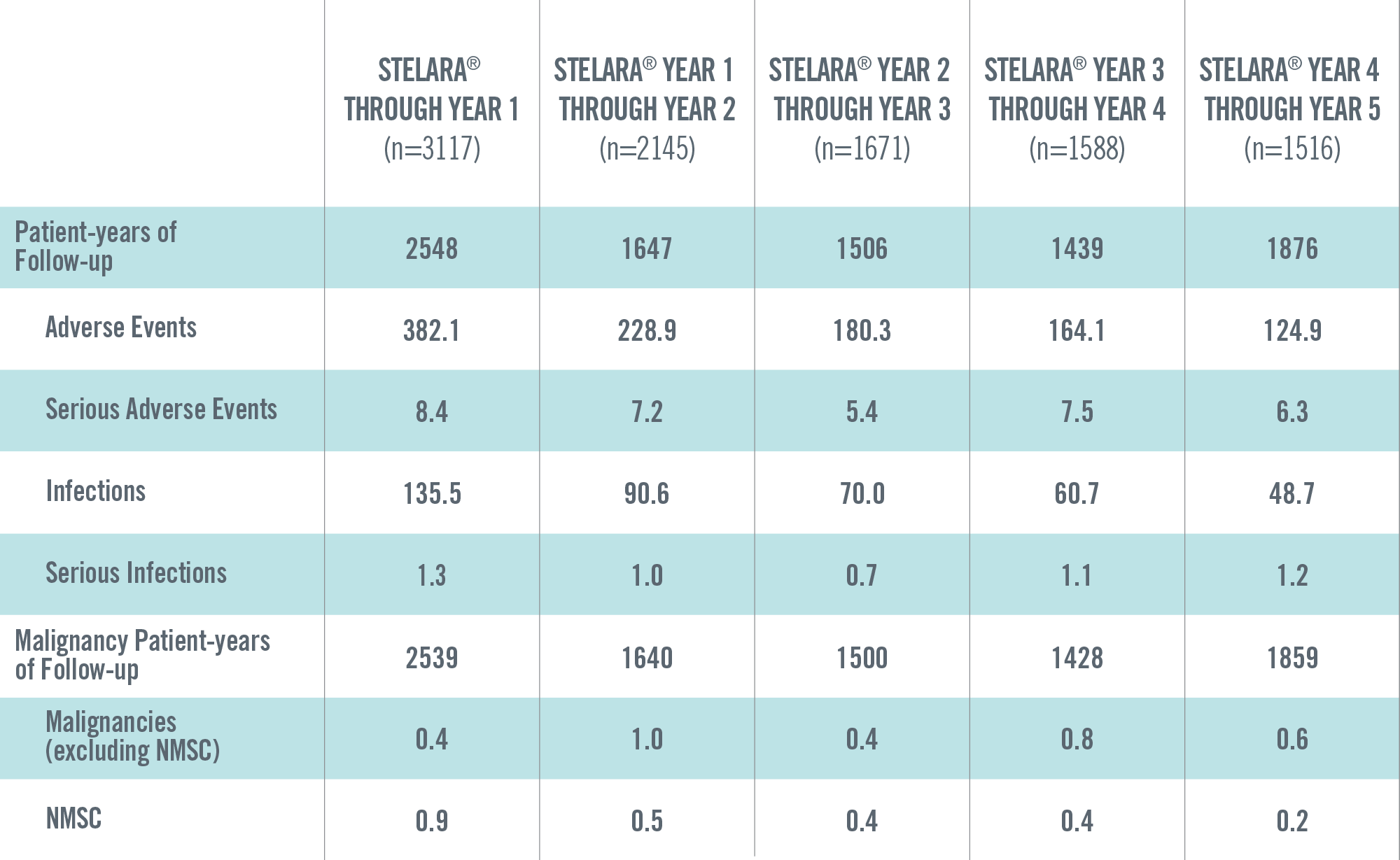
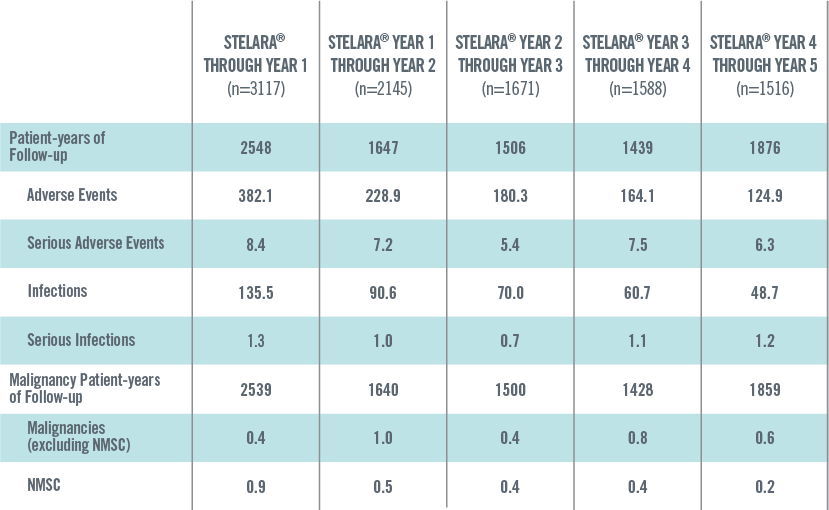
ADVERSE EVENTS, SERIOUS ADVERSE EVENTS, INFECTIONS, SERIOUS INFECTIONS AND MALIGNANCIES PER 100 PATIENT-YEARS THROUGH YEAR 5 IN STELARA® GLOBAL STUDIES 1*†
NMSC=Nonmelanoma Skin Cancer
*Includes Phase 2 (NCT00320216) and Phase 3 (PHOENIX 1, PHOENIX 2, ACCEPT) studies. These studies have varied exposure: in general, Year 1 reflects the data from all 4 studies, and Years 2 through 5 reflect the data primarily from PHOENIX 1 and PHOENIX 2.
†Year 1 is defined as ≤Week 48; Year 2 is defined as >Week 48 to ≤Week 96; Year 3 is defined as >Week 96 to ≤Week 144; Year 4 is defined as >Week 144 to ≤Week 192; Year 5 is defined as >Week 192.
Adverse Reactions Reported by ≥1% of Adult Patients Through Week 12 in PHOENIX 1 and PHOENIX 22
The safety data reflect exposure to STELARA® in 3117 adult psoriasis subjects, including 2414 exposed for at least 6 months, 1855 exposed for at least 1 year, 1653 exposed for at least 2 years, 1569 exposed for at least 3 years, 1482 exposed for at least 4 years and 838 exposed for at least 5 years.2
Events per 100 patient-years
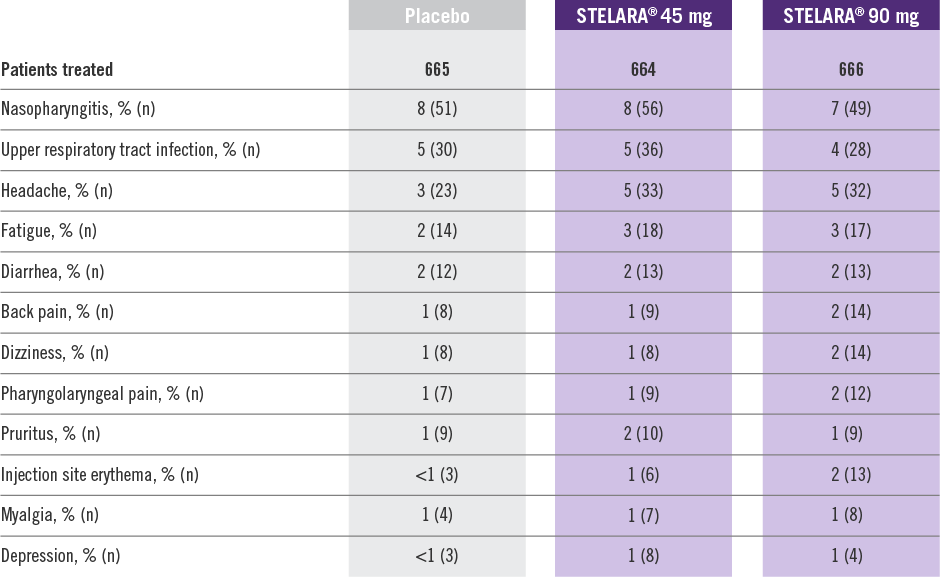
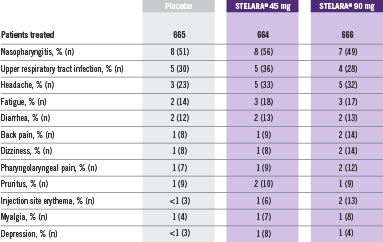
Adverse reactions that occurred in less than 1% in the controlled period of PHOENIX 1 and PHOENIX 2 through Week 12 included: cellulitis, herpes zoster, diverticulitis, and certain injection site reactions (pain, swelling, pruritus, induration, hemorrhage, bruising, and irritation).
One Case of Posterior Reversible Encephalopathy Syndrome (PRES) occurred during adult plaque psoriasis clinical studies.
RATE OF INFECTIONS AND MALIGNANCIES
In the placebo-controlled period of clinical studies of patients with psoriasis (average follow-up of 12.6 weeks for placebo-treated patients and 13.4 weeks for STELARA ®-treated patients)2:
27% of STELARA®-treated patients reported infections (1.39 per patient-year of follow-up) compared with 24% of placebo-treated patients (1.21 per patient-year of follow-up)
Serious infections occurred in 0.3% of STELARA®-treated patients (0.01 per patient-year of follow-up) and in 0.4% of placebo-treated patients (0.02 per patient-year of follow-up)
In the controlled and noncontrolled portions of psoriasis clinical trials (median follow-up of 3.2 years, representing 8998 patient-years of exposure)2:
72.3% of STELARA®-treated patients reported infections (0.87 per patient-year of follow-up)*
Serious infections were reported in 2.8% of patients (0.01 per patient-year of follow-up)
1.7% of STELARA®-treated patients reported malignancies excluding nonmelanoma skin cancers (NMSC) (0.60 per hundred patient-years of follow-up)
NMSC was reported in 1.5% of STELARA®-treated patients (0.52 per hundred patient-years of follow-up)
The most frequently observed malignancies other than NMSC during the clinical trials were prostate, melanoma, colorectal, and breast
Malignancies other than NMSC in STELARA®-treated patients during the controlled and noncontrolled portions of studies were similar in type and number to what would be expected in the general US population according to the Surveillance, Epidemiology, and End Results (SEER) database (adjusted for age, gender, and race). 2,3 †
Cumulative overall safety was evaluated during the placebo-controlled period (12 or 20 weeks) and up to 5 years of treatment.3
*Based on any adverse event, regardless of body system, which in the opinion of the investigator would be considered an infection.
†SEER database (2009), adjusted for age, gender, and race, compared with STELARA ® through the 2011 analysis.3
References: 1. Data on file. Janssen Biotech, Inc. 2. STELARA ® (ustekinumab) [package insert]. Horsham, PA: Janssen Biotech, Inc. 3. Papp KA, Griffiths CEM, Gordon K, et al; on behalf of the PHOENIX 1, PHOENIX 2, and ACCEPT Investigators. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol 2013;168(4):844-854.
cp-168045v3 05/23
