Click here for
supporting information
supporting information
*STELARA® is not approved for the treatment of scalp psoriasis.
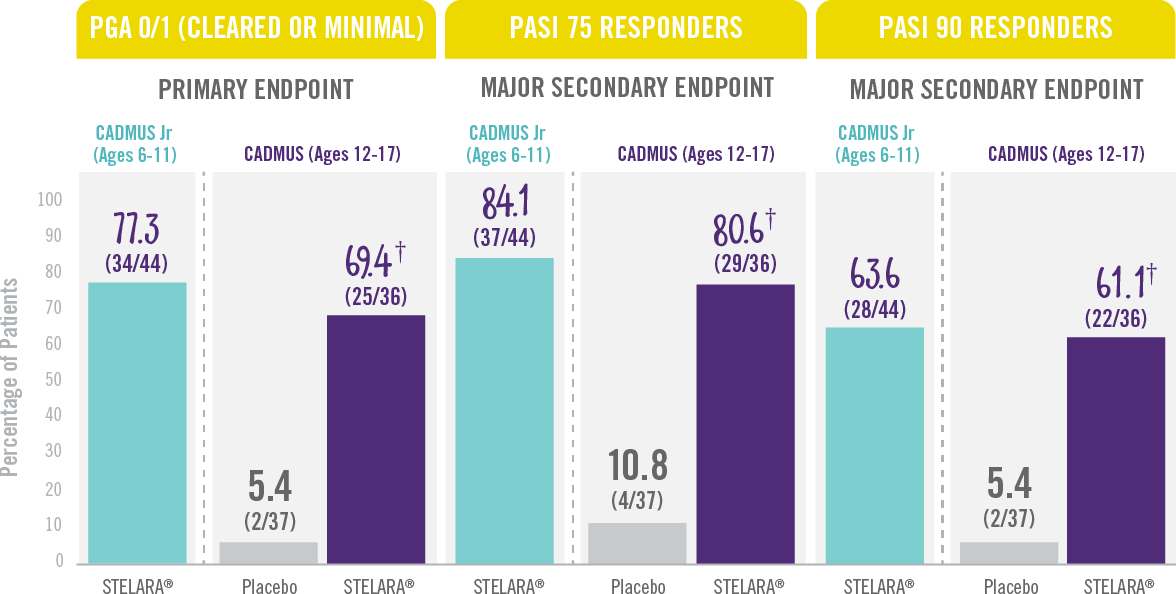
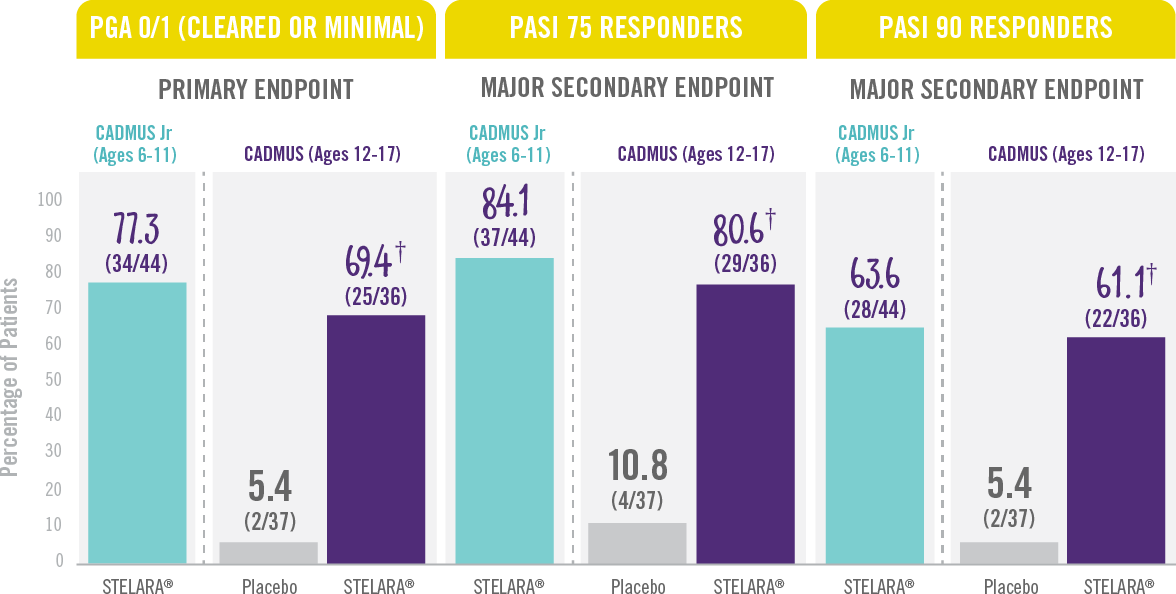
The majority of patients achieved a PGA score of cleared or minimal (primary endpoint) and PASI 75 and PASI 90 (secondary endpoints) at Week 12.†
For pediatric patients 6 years and older with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy
Skin Clearance at Week 12 With STELARA® After 2 Starter Doses1,2
RESULTS FROM 2 PHASE 3 TRIALS: CADMUS Jr (OPEN-LABEL) AND CADMUS (PLACEBO-CONTROLLED)*
† P<0.001 vs placebo.
*STELARA® efficacy results shown are from the standard-dosing arm of the CADMUS study.
†PGA=Physician’s Global Assessment. The PGA is a 6-category scale ranging from 0 (cleared) to 5 (severe) that indicates the physician’s overall assessment of psoriasis focusing on plaque thickness/induration, erythema, and scaling.
PASI 75= Proportion of patients who achieved 75% or more reduction (or improvement) in Psoriasis Area and Severity Index (PASI) score from baseline.
PASI 90=Proportion of patients who achieved 90% or more reduction (or improvement) in Psoriasis Area and Severity Index (PASI) score from baseline.
IN PEDIATRIC PATIENTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS
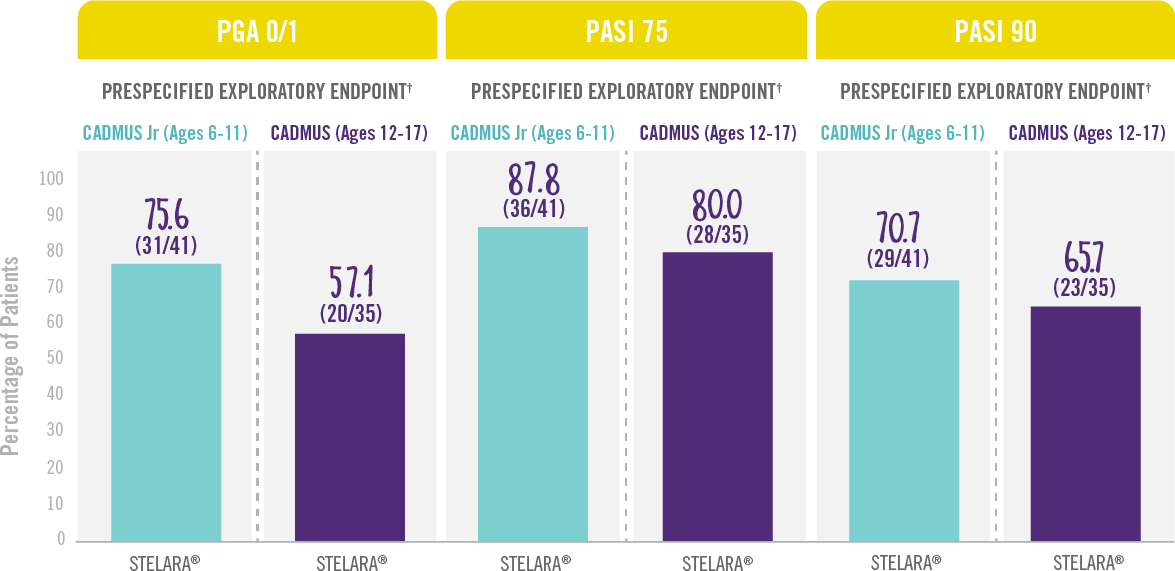
Skin Clearance Continued at Week 521,3
RESULTS FROM 2 PHASE 3 TRIALS: CADMUS Jr (OPEN-LABEL) AND CADMUS (PLACEBO-CONTROLLED)*
Treatment Failure Rules (TFR) were used for analysis. Patients who discontinued study treatment due to lack of efficacy, or an adverse event (AE) of worsening of psoriasis, or who started a protocol-prohibited medication were considered treatment failures.
*STELARA® efficacy results shown are from the standard-dosing arm of the CADMUS study.
†Prespecified exploratory endpoints: In CADMUS, during the blinded placebo-crossover and active-treatment period (Weeks 12 to 52), patients continued to receive STELARA® every 12 weeks through Week 40 with efficacy assessments continuing through Week 52. No formal statistical comparisons were performed at Week 52.
IN PEDIATRIC PATIENTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS
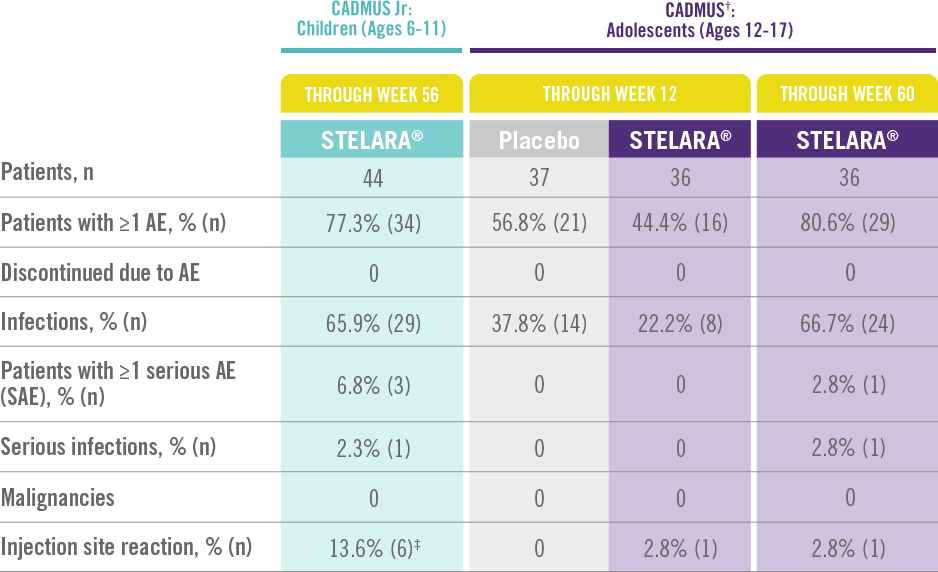
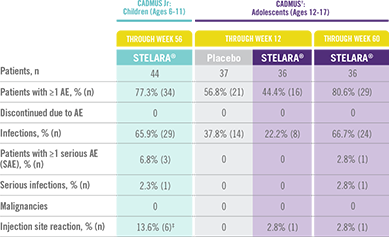
STELARA® Safety Profile Is Similar to Adult Studies1-3
ADVERSE EVENTS IN CADMUS Jr AND CADMUS STUDIES*
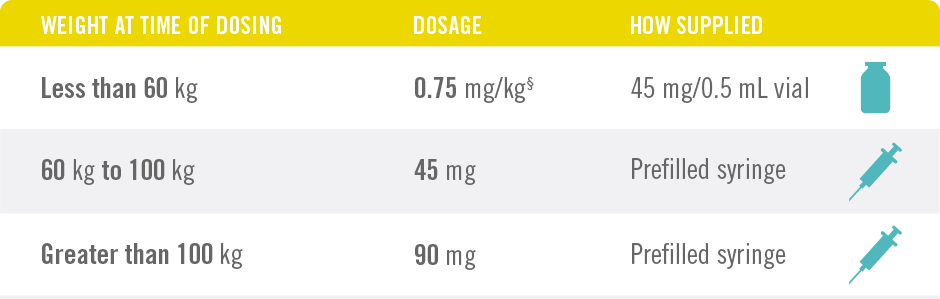
*STELARA® approved dose for pediatric patients is 0.75 mg/kg for patients weighing less than 60 kg; 45 mg for patients weighing 60 kg to 100 kg; and 90 mg for patients weighing greater than 100 kg administered subcutaneously at Weeks 0 and 4, and every 12 weeks thereafter.
†STELARA® safety results in the table above come from the standard-dosing arm of the CADMUS study.
‡All events were mild in intensity and resolved within 1 day.
The safety of STELARA® was assessed in a study of 44 patients from 6 to 11 years of age (CADMUS Jr) and in a study of 110 patients from 12 to 17 years of age (CADMUS) with moderate to severe plaque psoriasis. The safety profile in these patients through Week 56 or Week 60, respectively, was similar to the safety profile from studies in adults with plaque psoriasis1,2
In CADMUS Jr (patients ages 6 to 11), through Week 56, 29 (65.9%) patients reported 1 or more AEs that were considered infections by the investigator. The most common AEs that were considered to be infections were nasopharyngitis (11 [25.0%] subjects), pharyngitis (6 [13.6%] subjects), and upper respiratory tract infection (6 [13.6%] subjects)1
In CADMUS (patients ages 12 to 17), through Week 12, the most common AEs were nasopharyngitis (STELARA®, 2.8% [1/36]; placebo, 27.0% [10/37]) and headache (STELARA®, 8.3% [3/36]; placebo, 5.4% [2/37]). Through Week 60, the most common AEs across all treatment arms were nasopharyngitis (34.5% [38/110]), upper respiratory tract infection (12.7% [14/110]), and pharyngitis (8.2% [9/110])2,3
IN PEDIATRIC PATIENTS WITH MODERATE TO SEVERE PLAQUE PSORIASIS
Only 4 Doses of STELARA® per Year After 2 Starter Doses
STELARA® DOSING FOR PEDIATRIC PATIENTS BASED ON BODY WEIGHT, GIVEN SUBCUTANEOUSLY AT WEEKS 0 AND 4, THEN EVERY 12 WEEKS THEREAFTER4
§See Table 2 of the full Prescribing Information.
STELARA® is intended for use under the guidance and supervision of a physician. STELARA® should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician. The appropriate dose should be determined by a healthcare provider using the patient’s current weight at the time of dosing. In pediatric patients, it is recommended that STELARA® be administered by a healthcare provider.
FIRST 52 WEEKS OF THERAPY
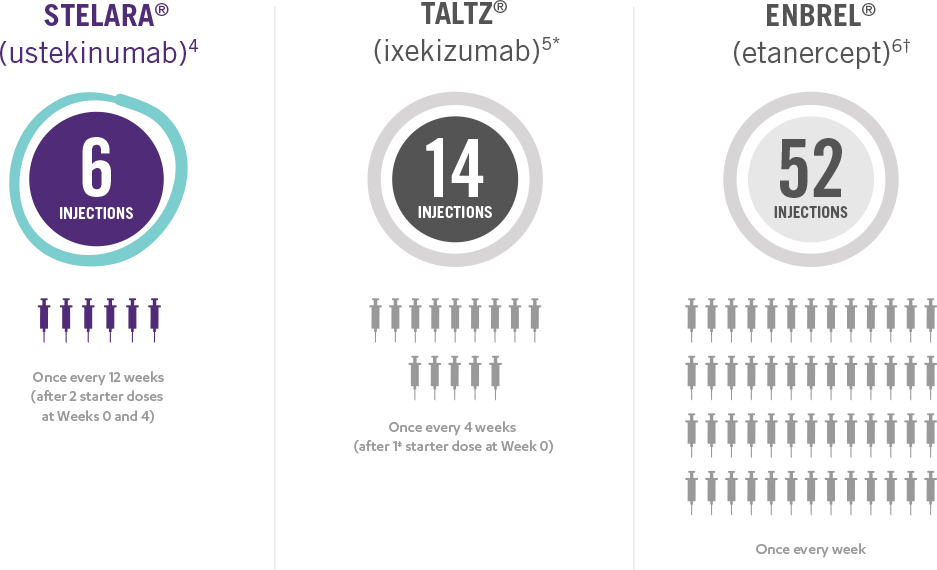
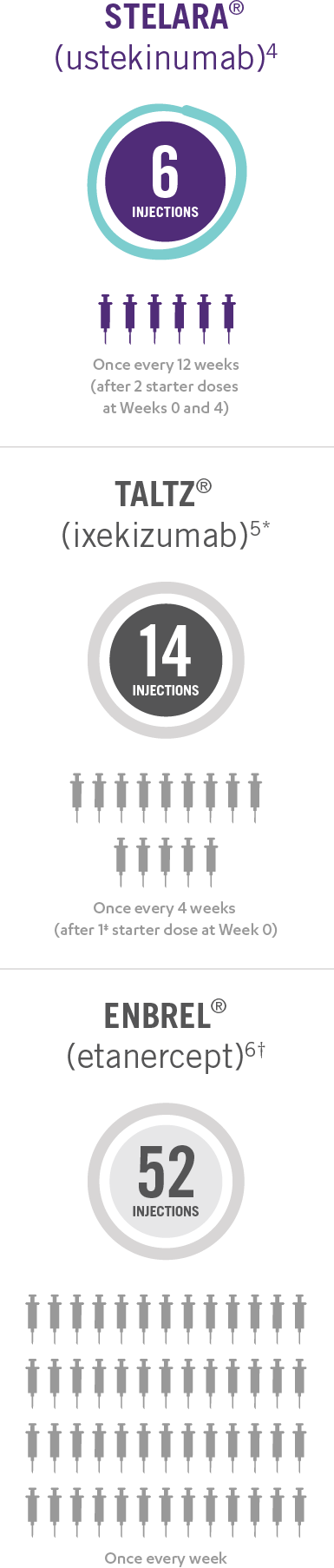
This presentation is not intended to compare the safety or efficacy of these treatments. Please refer to the full Prescribing Information of each product for complete dosage and administration information.
*Taltz® is for adults and children as young as 6 with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or phototherapy (treatment using ultraviolet or UV light).
Taltz® is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries and affiliates.
†Enbrel® is indicated for the treatment of patients 4 years of age or older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.
Enbrel® is a registered trademark of Amgen Inc.
‡Patients may require an additional starter dose depending on weight.
Find out how to HELP your pediatric patients requiring weight-based dosing WITH THE SINGLE-DOSE VIAL
References: 1. Philipp S , Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. [published online ahead of print March 16, 2020]. Br J Dermatol . doi: 10.1111/bjd.19018. 2. Landells I, Marano C, Hsu M-C, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73(4):594-603. 3. Data on file. Janssen Biotech, Inc. 4. STELARA® (ustekinumab) [prescribing information]. Horsham, PA: Janssen Biotech, Inc. 5. Taltz® (ixekizumab) [prescribing information]. Indianapolis, IN: Eli Lilly and Company. 6. Enbrel® (etanercept) [package insert]. Thousand Oaks, CA: Immunex Corporation.
cp-168045v4 11/25