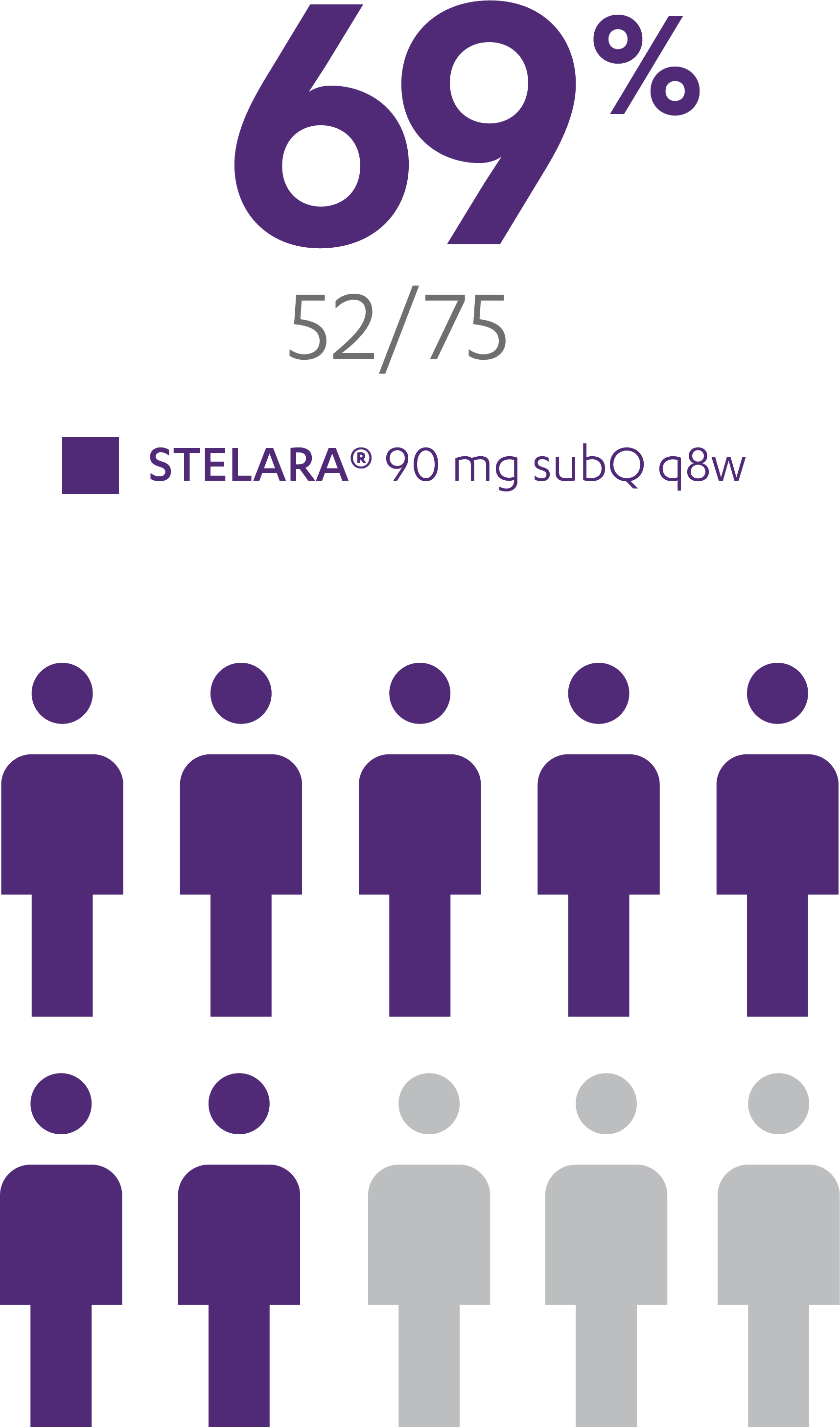
ANALYSIS RULES
Analyses shown are intent-to-treat:
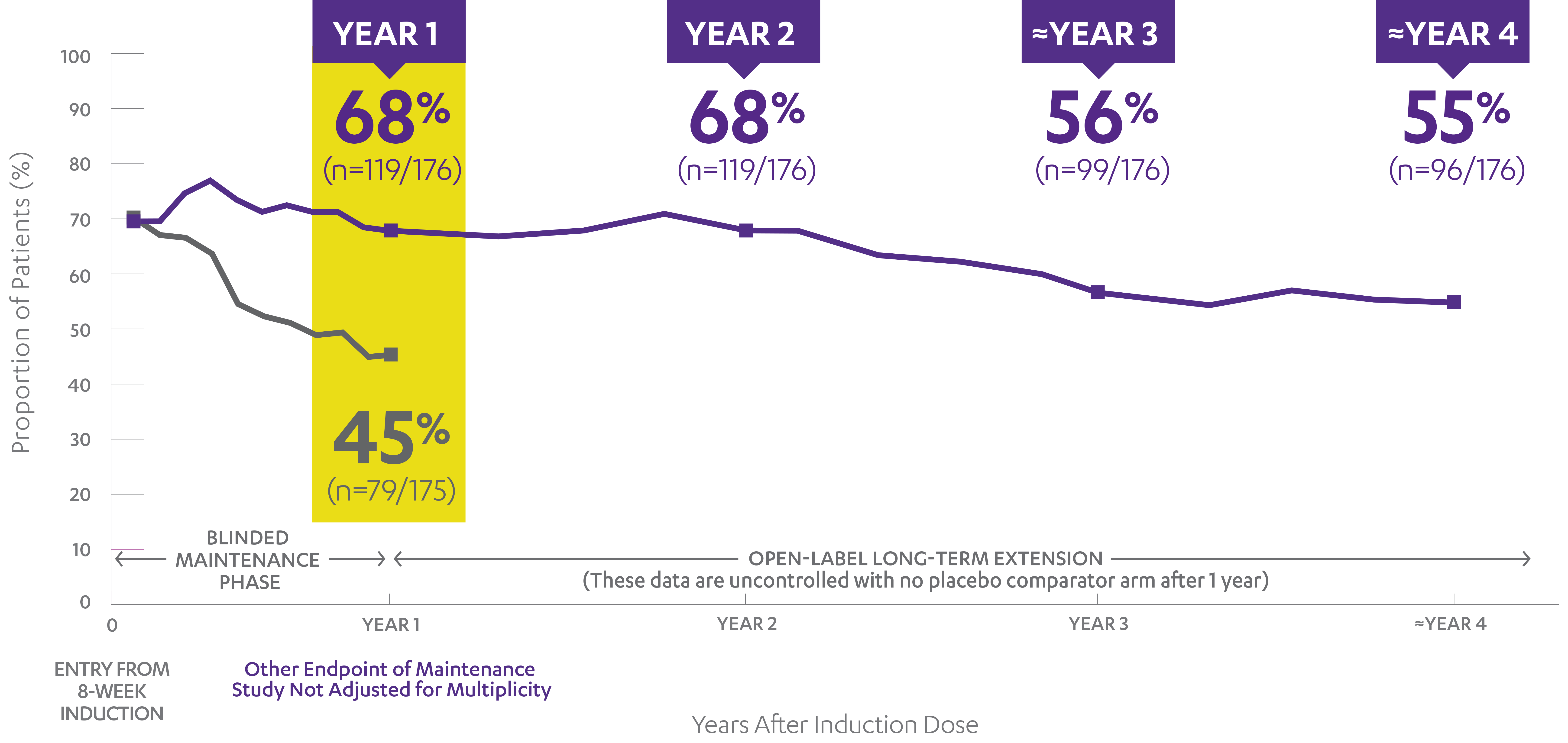
Patients who had a prohibited change in UC medication, an ostomy or colectomy, used a rescue medication after clinical flare, or discontinued study agent due to lack of therapeutic effect or due to an adverse event of worsening of UC prior to the Week 44 visit were considered not to be in symptomatic remission. Additionally, patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an adverse event of worsening of UC after Week 44 and prior to the designated visit, were considered not to be in symptomatic remission.
Patients who are receiving any of the UC-specific medical therapies (oral 5-ASA, AZA, 6-MP, or MTX) at the time of entry into the Maintenance Study and do not lose response must keep their prescribed dosage stable through completion of the Week 44 assessment. During the LTE, discontinuation of immunomodulators was allowed as determined by the investigator.